Figures & data
Figure 1. Overview of Injectable DDC Product Development Process and Typical Timelines in Relation to the Clinical Development Timeline.
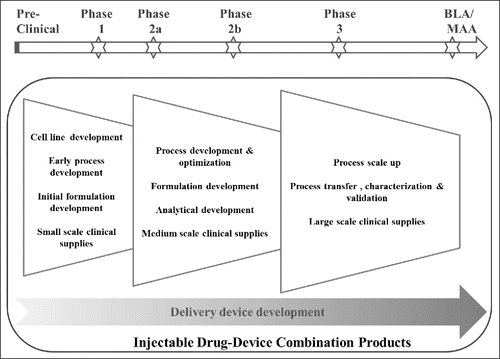
Figure 2. Nature and Timing of Biologics Manufacturing Process Changes Determines the Overall Potential Risk on Patient Safety and the Rigor of Comparability Assessment.
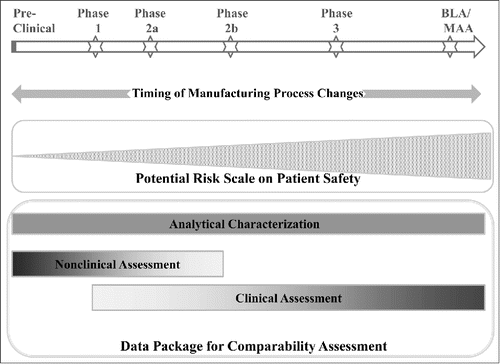
Table 1. List of FDA Approved Monoclonal Antibodies from January 2006 to March 2017 and the Clinical Comparability Assessment Studies to Support the Manufacturing Process Changes during Development Footnotea, b.
Table 2. Study Features of Human PK Comparability Trials to Support Manufacturing Process Changes in the FDA Database from January 2006 to March 2017.
Table 3. List of FDA Approved Monoclonal Antibodies DDC Products for SC Administration from January 2006 to March 2017: Clinical Studies in Support of Marketing Approvals of DDC Products.
Table 4. Clinical Studies with Usability-related Outcome Measurements for mAb DDC productsFootnotea.
