Figures & data
Table 1. The effect of fungal species in human health. The table indicates the fungal species causing diseases with references.
Table 2. Mycobiome tabulated data types. The table indicates references with fungal data types for discerning fungal genera.
Figure 1. Fundamental metabolic interactions between host-mycobiome in health and disease. A. indicates Candida contribution to the production of γ-amino butyric acid (GABA) through the tricarboxylic acid cycle to produce succinate may perceptively occur during systemic infection. GABA has a role in dampening the effect of the nervous system, and generally, deficiency involves epilepsy.Citation109 Indirectly targeting mycobiome in specific regions such as Candida species can have a better effect than providing analogues of GABA that has been identified to lead to anxiety, stress and seizures. Exploring this metabolic pathway of the mycobiome, especially targeting the succinate pathway, the production of semi-aldehydrase could lead to alteration of disease with better regulation from the microbiota, as alternatively, high levels of GABA have been linked to Alzheimer’s disease.Citation110 B. Penicillium species’ ability to produce the mycotoxin called citrinin is toxic to cause renal nephrosis.Citation111–113 Drugs have been developed to inhibit the effect of citrinin in hepatoma, and several animal studies have shown the benefit.Citation114 Ostry et al., 2013 have highlighted the dangerous effect of citrinin in dietary sources.Citation115 There has been no established link of mycobiome being the contributor of toxin in humans and whether diverting the polyketide pathway can reduce renal cell toxicity. C Aflatoxin is a common secondary metabolite of the Aspergillus family. It has been associated with cancer morbidity and inducing tumour suppressor gene p53.Citation116–119 The potential effect of the presence of Aspergillus needs to be investigated and whether manipulating the metabolic pathway can give a better indicator of being able to reverse the effects of aflatoxin by reducing the levels of the malonyl-coA pathway. All these potential pathways give us a clear indication of how the study of the metabolism of the mycobiome is essential in understanding pathogenesis across a wide breadth of diseases.
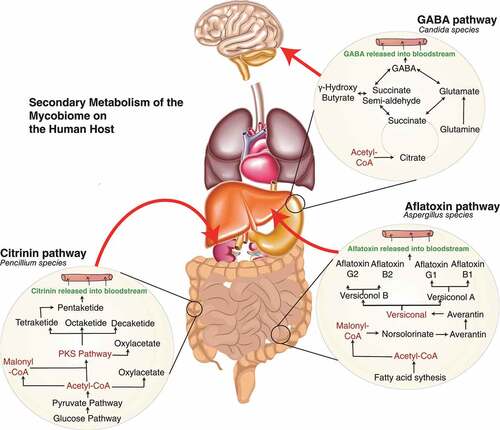
Table 3. Highlight the effect of metabolites on the host. Identification of a wide variety of secondary metabolites from various fungal species, the class grouping attributed to the metabolite, based upon the synthesis, fungal species responsible for chemically engineering the metabolite and the effect on the human host. The properties include anti-bacterial activity to cancerous effect. The extended table includes the effect on the environment, marine biology and produce. *IL = interleukin ** blue highlight indicates human effect.
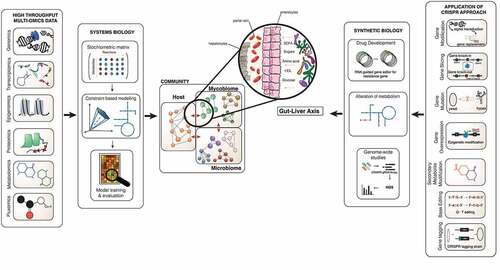
Figure 2. System and synthetic biology approach in investigating mycobiome’s important role in the gut-liver axis. This image shows the interaction of the microbiome-mycobiome with the host cells in the gut, translocation of intermediaries through the blood, and prospectively leading to the composition of the liver environment to change. The biological knowledge from the collection of omics data such as proteome, metabolome and transcriptome generates information that can be integrated into functional mathematical models. The structural data is placed into the stoichiometric matrix and after several steps of adding biochemical information the draft of the GSMM can be reconstructed and further validated for new biological interpretation and predictions. Different constraint can be applied on the GSMMs to investigate the models for novel discovery. CRISPR as one of the gene editing approaches in validating predictions based on GSMM; these can be done using the CRISPR-cas9 system to perform genome modification such as gene slicing, gene mutation, secondary metabolite modification, base editing, and gene editing tagging and gene overexpression, down-regulation methods. The application can be used in tackling drug resistance, alteration of the metabolic pathway with the possibility of amending toxic pathways in pathogenesis, rationalising gene essentiality and genome-wide screening. The application of these potential methods in assessing the mycobiome community and particularly within the gut-liver axis.