Figures & data
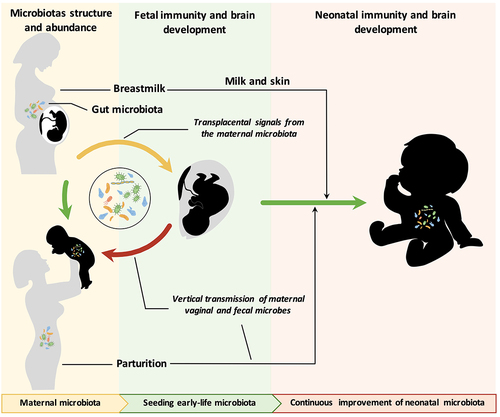
Figure 1. The maternal microbiota and its metabolites regulate fetal development before birth and during lactation. The maternal gut, breast milk and vaginal microbiota are major factors regulating offspring development. The maternal microbiome is influenced by a variety of factors, including age, genetics, diet, body weight, delivery mode, medication use, and the environment. The effects of various factors on the maternal microbiome may be passed on to the offspring through microbes, microbial products or metabolites, which regulate early gut colonization, immune maturation, and brain development in infants.
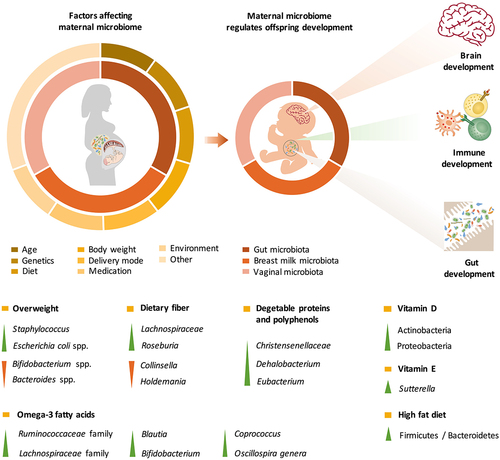
Figure 2. Mother-to-offspring bacterial transmission during pregnancy and lactation. (a) Gut-placental transmission hypothesis. This hypothesis states that transmission of the maternal microbiome to the fetus occurs before birth. Bacteria from the maternal intestine can be harvested by dendritic cells and then migrate to the fetus through the blood. (b) Enteromammary transfer hypothesis. Breast milk functions as the most important postnatal link between infants and mothers. In addition to macronutrients, micronutrients, oligosaccharides and immune factors, maternal milk contains a microbiota. This hypothesis states that the parent microbes combine with IgA in the gut to form a complex (IgA+). IgA+ plasma cells translocate to maternal mammary tissue via intestinal Peyer’s patches (PPs) to release microorganisms and secrete IgA. Breastfeeding newborns acquire nutrients and microorganisms in milk. Microorganisms in milk are transferred to colonize the neonatal gut. (c) Retrograde flow transfer hypothesis. This hypothesis proposes that the microbiota from the maternal skin, fetal oral cavity and environment enter the maternal mammary gland during lactation, thereby composing the milk microbial community.
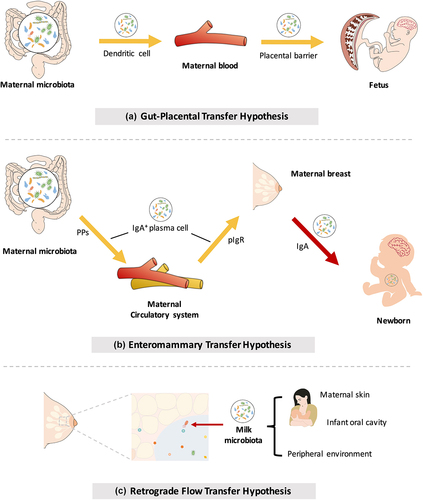
Figure 3. The maternal microbiota or microbiota metabolites regulate fetal immunity and brain development. Bacterial metabolites can be absorbed into the bloodstream and then transported to the fetus during placentation. These metabolites derived from maternal bacteria regulate multiple biological functions of the fetus. Short-chain fatty acids (SCFAs) produced by microbial fermentation of high-fiber diets may regulate microglial maturation, microglial cell-neuron interactions, and synaptic function in the hippocampus by activating fatty acid receptors (GPR41 and GPR43). During pregnancy, maternal microbes also increase axonal numbers and promote thalamocortical axon development in the fetal brain by modulating the levels of metabolites, such as trimethylamine-N-oxide (TMAO) and imidazole propionate (IP). In addition, pathogen infection during pregnancy increases proinflammatory cytokine IL-17a secretion, which is transmitted through the circulation, affects fetal brain development and induces abnormal cortical phenotypes. Maternal microbes are also involved in the regulation of fetal immune establishment during pregnancy. Maternal intestinal Escherichia coli HA107 colonization increases fetal intestinal leukocyte proliferation (that of type 3 innate lymphocytes (Ilc3s) and monocytes) as well as the production of intestinal antimicrobial peptides (C-type lectins and defensins of the REG family) and mucus. Some microbiota-derived compounds are microbial aryl hydrocarbon receptor (AhR) ligands, which promote the amplification of ILC3s. Maternal-derived SCFAs also regulate thymus and T-lymphocyte development. Maternal infection by a pathogen (Yersinia pseudotuberculosis) regulates fetal immunity through an increase in the levels of IL-6, which crosses the placental barrier and promotes intestinal Th17 cell responses and intestinal protective immunity in the fetus. Therefore, maternal microbial metabolites (AhR ligands and SCFAs) and the cytokine-mediated response (IL-6) synergistically regulate the early establishment of immunity before birth. AIRE, autoimmune regulator; AJ, adherens junction; GPCRs, G protein-coupled receptors; GVB, gut vascular barrier; IFN-γ, interferon γ; IgA, immunoglobulin A; IgA+, IgA+ plasma cells; IgG, immunoglobulin G; pIgr, polymeric immunoglobulin receptor; TJ, tight junction.
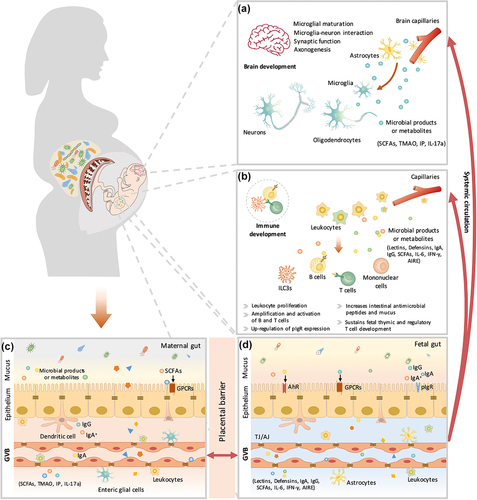
Table 1. Effect of maternal probiotic and prebiotic supplementation during pregnancy on brain development in offspring.
Table 2. Effect of maternal probiotic and prebiotic supplementation during pregnancy on immune development in offspring.
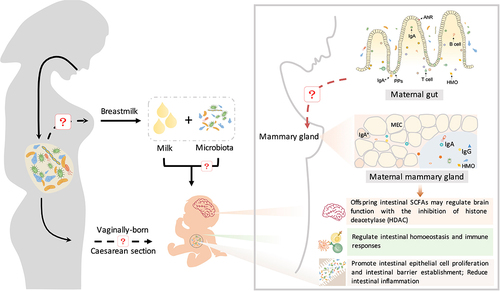
Figure 4. The maternal microbiota regulates neonatal immunity and brain development. Beneficial microbes inherited from breast milk contribute to the establishment of the immune system in neonates. Beneficial microbes from breast milk promote the production of immunoglobulins (IgA and IgG) and regulate intestinal homeostasis and immune responses (CD4+ T cells and monocytes) through the aryl hydrocarbon receptor (AhR). IgA+ plasma cells are recruited from maternal intestinal Peyer’s patches (PPs) and then translocate through the circulatory system to the mammary gland and secrete IgA. Furthermore, maternal intestinal Ig-coated bacteria can directly translocate to the mammary gland and then be enriched in maternal milk. Immunoglobulins in milk promote the differentiation of neonatal intestinal epithelial cells and the establishment of intestinal barrier function, reducing intestinal inflammation. During this process, immunoglobulins cooperate with gut microbes to regulate innate and adaptive immunity in neonates. In addition, maternal prebiotic intake increases the concentrations of offspring intestinal SCFAs, which may cross the blood – brain barrier and modulate brain function by inhibiting histone deacetylases (HDACs).