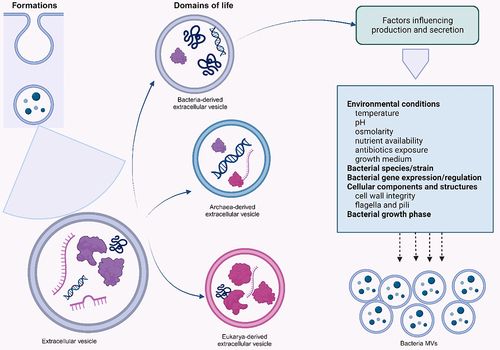
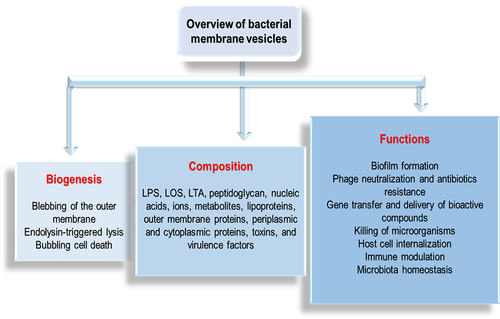
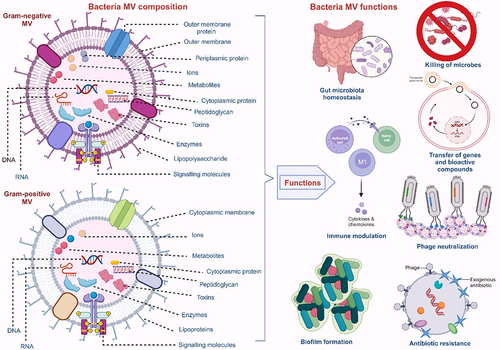
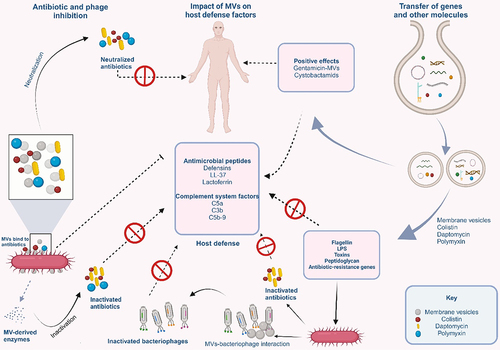
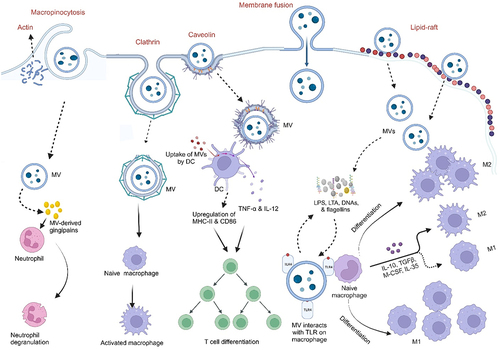
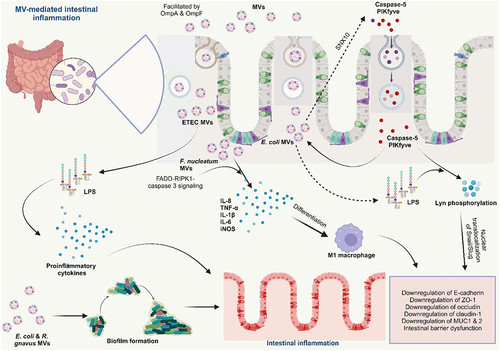
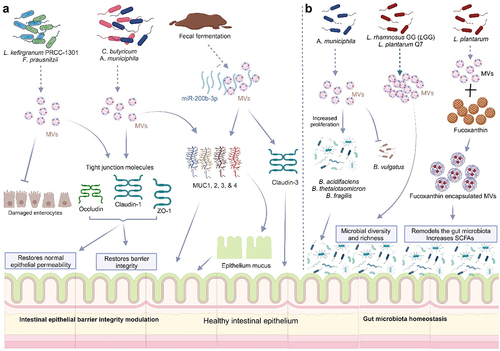
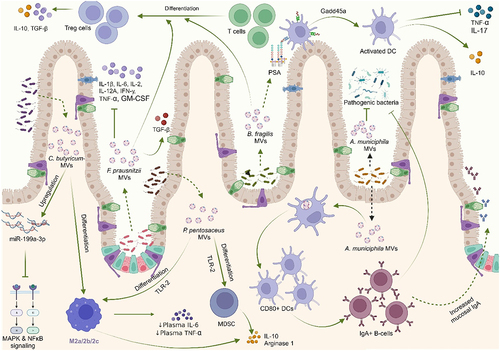
Figures & data
Table 1. Differences between MVs produced by Gram-negative bacteria and Gram-positive bacteria.
Table 2. Bacterial MVs in the pathogenesis of IBD.
Table 3. Application of MVs in IBD therapy.
Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol [Internet]. 2015;13(10):605–619. doi: 10.1038/nrmicro3525. Bose S, Aggarwal S, Singh DV, Acharya N. Extracellular vesicles: An emerging platform in gram-positive bacteria. Microb Cell [Internet]. 2020;7(12):312–322. doi:10.15698/mic2020.12.737. Sartorio MG, Pardue EJ, Feldman MF, Haurat MF. Bacterial outer membrane vesicles: from discovery to applications. Annu Rev Microbiol [Internet]. 2021;75(1):609–630. doi:10.1146/annurev-micro-052821-031444. Toyofuku M, Schild S, Kaparakis-Liaskos M, Eberl L. Composition and functions of bacterial membrane vesicles. Nat Rev Microbiol [Internet]. 2023;21(7):415–430. doi:10.1038/s41579-023-00875-5. Yu Y, Wang X, Fan G. Versatile effects of bacterium-released membrane vesicles on mammalian cells and infectious/inflammatory diseases. Acta Pharmacol Sin [Internet]. 2017;39(4):514–533. https://www.semanticscholar.org/paper/9a0f9369ab7f811db5dc161c47d5c99fe1d375a6. Brown L, Wolf JM, Prados-Rosales R, Casadevall A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol [Internet]. 2015;13(10):620–630. doi:10.1038/nrmicro3480. Liu Y, Defourny KAY, Smid EJ, Abee T. Gram-positive bacterial extracellular vesicles and their impact on health and disease. Front Microbiol [Internet]. 2018;9:385261. doi:10.3389/fmicb.2018.01502/full. Briaud P, Carroll RK, Richardson AR. Extracellular vesicle biogenesis and functions in gram-positive bacteria. Infect Immun. 2020;88(12):10–128. doi:10.1128/IAI.00433-20. Kulp A, Kuehn MJ. NIH Public Access. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annual Review of Microbiology. 2010;64:163–184. doi:10.1146/annurev.micro.091208.073413. Kim JH, Lee J, Park J, Gho YS. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin Cell Dev Biol [Internet]. 2015;40:97–104. https://linkinghub.elsevier.com/retrieve/pii/S1084952115000336. Toyofuku M, Nomura N, Eberl L. Types and origins of bacterial membrane vesicles. Nat Rev Microbiol [Internet]. 2019;17(1):13–24. doi:10.1038/s41579-018-0112-2. Gan Y, Zhao G, Wang Z, Zhang X, Wu MX, Lu M. Bacterial membrane vesicles: physiological roles, infection immunology, and applications. Adv Sci [Internet]. 2023;10(25). doi:10.1002/advs.202301357. Toyofuku M, Cárcamo-Oyarce G, Yamamoto T, Eisenstein F, Hsiao C-C, Kurosawa M, Gademann K, Pilhofer M, Nomura N, Eberl L. Prophage-triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis. Nat Commun [Internet]. 2017;8(1):481. doi:10.1038/s41467-017-00492-w. Schlatterer K, Beck C, Hanzelmann D, Lebtig M, Fehrenbacher B, Schaller M, Ebner P, Nega M, Otto M, Kretschmer D, et al. The mechanism behind bacterial lipoprotein release: phenol-soluble modulins mediate toll-like receptor 2 activation via extracellular vesicle release from staphylococcus aureus. MBio [Internet]. 2018;9(6). doi:10.1128/mBio.01851-18. Hickey CA, Kuhn KA, Donermeyer DL, Porter NT, Jin C, Cameron EA, Jung H, Kaiko GE, Wegorzewska M, Malvin NP, et al. Colitogenic bacteroides thetaiotaomicron antigens access host immune cells in a sulfatase-dependent manner via outer membrane vesicles. Cell Host Microbe [Internet]. 2015;17(5):672–680. https://linkinghub.elsevier.com/retrieve/pii/S1931312815001602. Thapa HB, Kohl P, Zingl FG, Fleischhacker D, Wolinski H, Kufer TA, Schild S, Josenhans C. Characterization of the inflammatory response evoked by bacterial membrane vesicles in intestinal cells reveals an RIPK2-dependent activation by enterotoxigenic escherichia coli vesicles. Microbiol Spectr. 2023;11(4):11. doi:10.1128/spectrum.01115-23. Kunsmann L, Rüter C, Bauwens A, Greune L, Glüder M, Kemper B, Fruth A, Wai SN, He X, Lloubes R, et al. Virulence from vesicles: Novel mechanisms of host cell injury by Escherichia coli O104: H4 outbreak strain. Sci Rep [Internet]. 2015;5(1):13252.https://www.nature.com/articles/srep13252. Rolhion N, Barnich N, Claret L, Darfeuille-Michaud A. Strong decrease in invasive ability and outer membrane vesicle release in crohn’s disease-associated adherent-invasive escherichia coli strain LF82 with the yfgL gene deleted. J Bacteriol [Internet]. 2005;187:2286–2296. doi:10.1128/JB.187.7.2286-2296.2005. Wang X, Ni J, You Y, Feng G, Zhang S, Bao W, Hou H, Li H, Liu L, Zheng M, et al. SNX10‐mediated LPS sensing causes intestinal barrier dysfunction via a caspase‐5‐dependent signaling cascade. Embo J [Internet]. 2021;40(24): doi:10.15252/embj.2021108080. Engevik MA, Danhof HA, Ruan W, Engevik AC, Chang-Graham AL, Engevik KA, Shi Z, Zhao Y, Brand CK, Krystofiak ES, et al. Fusobacterium nucleatum secretes outer membrane vesicles and promotes intestinal inflammation. MBio [Internet]. 2021;12(2):10–128. doi:10.1128/mBio.02706-20. Liu L, Liang L, Yang C, Zhou Y, Chen Y. Extracellular vesicles of Fusobacterium nucleatum compromise intestinal barrier through targeting RIPK1-mediated cell death pathway. Gut Microbes [Internet]. 2021;13(1). doi:10.1080/19490976.2021.1902718. Wei S, Zhang J, Wu X, Chen M, Huang H, Zeng S, Xiang Z, Li X, Dong W. Fusobacterium nucleatum extracellular vesicles promote experimental colitis by modulating autophagy via the miR-574-5p/CARD3 axis. Inflamm Bowel Dis [Internet]. 2023;29(1):9–26. https://academic.oup.com/ibdjournal/article/29/1/9/6674006. Pavkova I, Klimentova J, Bavlovic J, Horcickova L, Kubelkova K, Vlcak E, Raabova H, Filimonenko V, Ballek O, Stulik J. Francisella tularensis outer membrane vesicles participate in the early phase of interaction with macrophages. Front Microbiol [Internet]. 2021;12:748706. doi:10.3389/fmicb.2021.748706/full. Kuhn T, Koch M, Fuhrmann G. Probiomimetics—novel lactobacillus ‐mimicking microparticles show anti‐inflammatory and barrier‐protecting effects in gastrointestinal models. Small [Internet]. 2020;16(40). doi:10.1002/smll.202003158. Kang EA, Choi H-I, Hong SW, Kang S, Jegal H-Y, Choi EW, Park B-S, Kim JS. Extracellular vesicles derived from kefir grain lactobacillus ameliorate intestinal inflammation via regulation of proinflammatory pathway and tight junction integrity. Biomedicines Int [Internet]. 2020;8(11):522. Ma L, Shen Q, Lyu W, Lv L, Wang W, Yu M, Yang H, Tao S, Xiao Y, Claesen J. Clostridium butyricum and its derived extracellular vesicles modulate gut homeostasis and ameliorate acute experimental colitis. Microbiol Spectr. 2022;10(4). doi:10.1128/spectrum.01368-22. Liang L, Yang C, Liu L, Mai G, Li H, Wu L, Jin M, Chen Y. Commensal bacteria-derived extracellular vesicles suppress ulcerative colitis through regulating the macrophages polarization and remodeling the gut microbiota. Microb Cell Fact [Internet]. 2022;21(1):88. doi:10.1186/s12934-022-01812-6. Wang X, Lin S, Wang L, Cao Z, Zhang M, Zhang Y, Liu R, Liu J. Versatility of bacterial outer membrane vesicles in regulating intestinal homeostasis. Sci Adv [Internet]. 2023;9(11). doi:10.1126/sciadv.ade5079. Kang C, Ban M, Choi E-J, Moon H-G, Jeon J-S, Kim D-K, Park S-K, Jeon SG, Roh T-Y, Myung S-J, et al. Extracellular vesicles derived from gut microbiota, especially akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLOS One [Internet]. 2013;8(10):e76520. doi:10.1371/journal.pone.0076520. Fábrega M-J, Rodríguez-Nogales A, Garrido-Mesa J, Algieri F, Badía J, Giménez R, Gálvez J, Baldomà L. Intestinal anti-inflammatory effects of outer membrane vesicles from escherichia coli nissle 1917 in DSS-experimental colitis in Mice. Front Microbiol [Internet]. 2017;8. doi:10.3389/fmicb.2017.01274/full. Choi JH, Moon CM, Shin T-S, Kim EK, McDowell A, Jo M-K, Joo YH, Kim S-E, Jung H-K, Shim K-N, et al. Lactobacillus paracasei-derived extracellular vesicles attenuate the intestinal inflammatory response by augmenting the endoplasmic reticulum stress pathway. Exp Mol Med [Internet]. 2020;52(3):423–437. http://www.nature.com/articles/s12276-019-0359-3. Tong L, Zhang X, Hao H, Liu Q, Zhou Z, Liang X, Liu T, Gong P, Zhang L, Zhai Z, et al. Lactobacillus rhamnosus GG derived extracellular vesicles modulate gut microbiota and attenuate inflammatory in DSS-induced colitis mice. Nutr [Internet]. 2021;13(10):3319. doi:10.3390/nu13103319. Hao H, Zhang X, Tong L, Liu Q, Liang X, Bu Y, Gong P, Liu T, Zhang L, Xia Y, et al. Effect of extracellular vesicles derived from lactobacillus plantarum Q7 on gut microbiota and ulcerative colitis in mice. Front Immunol [Internet]. 2021;12. doi:10.3389/fimmu.2021.777147/full. Liang D, Liu C, Li Y, Wu C, Chen Y, Tan M, Su W. Engineering fucoxanthin-loaded probiotics’ membrane vesicles for the dietary intervention of colitis. Biomaterials [Internet]. 2023;297:122107. https://linkinghub.elsevier.com/retrieve/pii/S0142961223001151. Shen Y, Torchia MLG, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe [Internet]. 2012;12(4):509–520. https://linkinghub.elsevier.com/retrieve/pii/S1931312812002752. Rodovalho VDR, Luz BD, Rabah H, Do Carmo FLR, Folador EL, Nicolas A, Jardin J, Briard-Bion V, Blottière H, Lapaque N, et al. Extracellular vesicles produced by the probiotic propionibacterium freudenreichii CIRM-BIA 129 mitigate inflammation by modulating the NF-κB pathway. Front Microbiol [Internet]. 2020;11:11. doi:10.3389/fmicb.2020.01544/full. Alpdundar Bulut E, Bayyurt Kocabas B, Yazar V, Aykut G, Guler U, Salih B, Surucu Yilmaz N, Ayanoglu IC, Polat MM, Akcali KC, et al. Human gut commensal membrane vesicles modulate inflammation by generating m2-like macrophages and myeloid-derived suppressor Cells. J Immunol [Internet]. 2020;205(10):2707–2718.https://journals.aai.org/jimmunol/article/205/10/2707/107686/Human-Gut-Commensal-Membrane-Vesicles-Modulate. Fonseca S, Carvalho AL, Miquel-Clopés A, Jones EJ, Juodeikis R, Stentz R, Carding SR. Extracellular vesicles produced by the human gut commensal bacterium Bacteroides thetaiotaomicron elicit anti-inflammatory responses from innate immune cells. Front Microbiol [Internet]. 2022;13:1050271. doi:10.3389/fmicb.2022.1050271/full. Ye L, Wang Y, Xiao F, Wang X, Li X, Cao R, Zhang J, Zhang T. F. prausnitzii-derived extracellular vesicles attenuate experimental colitis by regulating intestinal homeostasis in mice. Microb Cell Fact [Internet]. 2023;22(1):235. doi:10.1186/s12934-023-02243-7.