Figures & data
Figure 1. Double Immunofluorescence for epithelial cell line A549 and alveolar macrophages murine AMJ2-C11. (A) Uninfected A549 cells. (B) A549 cells infected with P. brasiliensis. A549 cells were labeled with FITC-phalloidin (green), P. brasiliensis stained with anti-cell free antibody and Alexa Fluor® 594 conjugate and nucleus was labeled with DAPI (blue). (C) Uninfected macrophages. (D) Macrophages infected with P. brasiliensis. Macrophages were immunolabeled with the primary antibodies anti- cytoplasmic protein and secondary conjugated Alexa Fluor R 488 (green), and P. brasiliensis immunolabeled with anti-cell-free antibody (red) and secondary conjugated Alexa Fluor ® 594, and nucleus was labeled with DAPI (blue) (Zeiss LSM 510 Meta Confocal Microscope).
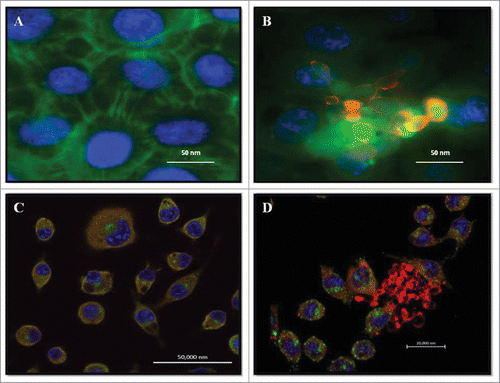
Figure 2. Kinetics of biofilm formation by P. brasiliensis in microdilution plates. (A) Measurements determined by the XTT reduction assay. Each point represents the mean of 3 measurements of absorbance at 490nm on a microtiter reader (iMarkTM Microplate Reader; BIO-RAD). (B) Kinetics monitored by Microphotograph taken using a camera attached to an inverted microscope. Bars = 30 nm for all panels.
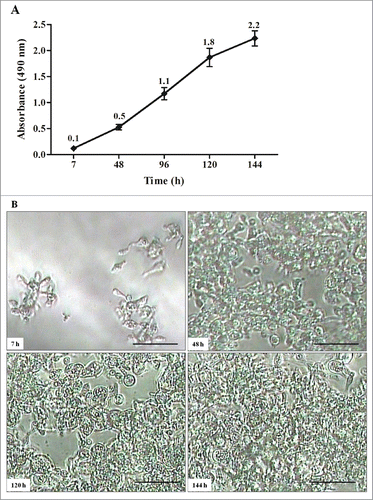
Figure 3. Images of mature biofilms of P. brasiliensis (Pb 18), formed after incubation for 144 h at 37°C 40× magnification. Images were acquired by Fluorescence microscopy and the fungi biofilms were stained by Calcofluor White Stain reagent (Fluka ®). All scale bars are 125 μm.
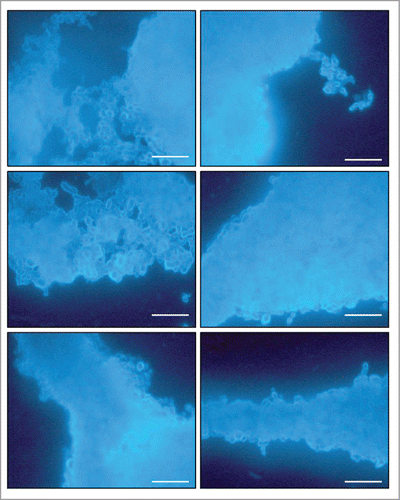
Figure 4. SEM images of mature biofilms of P. brasiliensis (Pb 18), in different dimensions, formed after incubation for 144 h at 37°C (A–D). In the arrows in showing the presence of extracellular matrix.
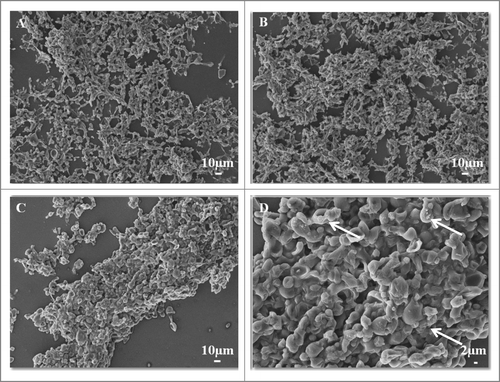
Figure 5. CLSM images of mature biofilms of P. brasiliensis (144 h). Fluorescence labeling of P. brasiliensis biofilms. (A and C) Biofilm was immunolabeled with primary antibodies anti-cell-free and secondary conjugated Alexa Fluor ® 488. (B) Scale depth image A showing the thickness of the biofilm. (D) Projection of biofilm formation of P. brasiliensis 2.5D (Zeiss LSM 510 Meta Confocal Microscope).
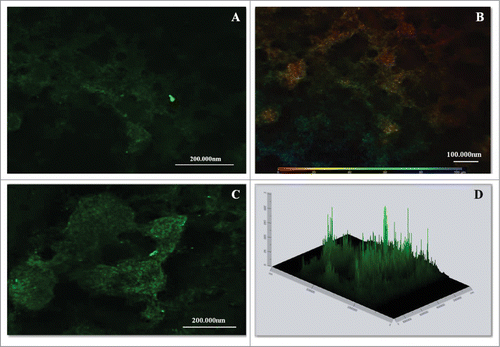
Figure 6. Relative expression of adhesins/ enzymes of P. brasiliensis during growth in biofilm and planktonic conditons, both in low oxygen tension using real-time PCR (qRT-PCR). Statistical analyzes were performed by Student's t-test, P < 0.05.
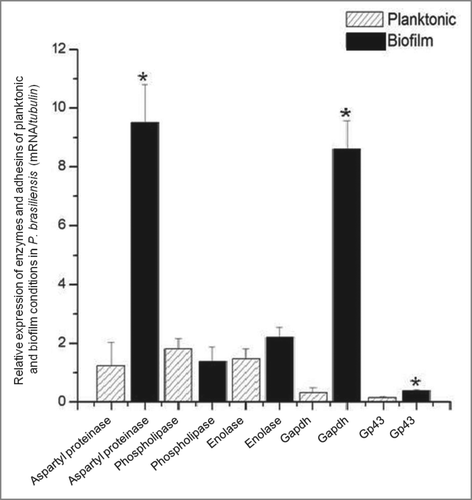
Table 1 Primers utilized in this study

