Figures & data
Figure 1. Phenotype of antiCD44v6 CAR+.CIK cells. Mature CIK cells efficiently expressed anti-CD44v6CAR (A). The CD3+CD56+ subset of CIK cells was equally distributed between CAR+ (B) and CAR− (C) cells. CAR+.CIK were mostly effector memory (EM / EM RA), followed in order by naive and central memory phenotype (D). The phenotype was comparable with that of unmodified NTD.CIK (unpaired t test, P > 0.05). Abbreviations: Chimeric Antigen Receptor (CAR); Not Transduced (NTD); Effector Memory (EM: CD45RA−/CD62 L-); Effector Memory RA (EM RA: CD45RA+/CD62 L-); Central Memory (CM: CD45RA−/CD62 L+); NAÏVE: CD45RA+/CD62 L+.
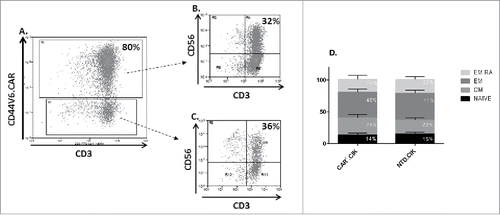
Table 1. CD44v6 expression in STS.
Table 2. Phenotype of STS cell lines.
Figure 2. Sarcoma killing in vitro by antiCD44v6 CAR+.CIK Patient-derived antiCD44v6 CAR+.CIK (n = 12) efficiently killed STS (n = 11, autologous targets in 4/11). The specific killing by CAR+.CIK cells was significantly higher, especially at low E/T ratios, than that obtained with unmodified CIK generated from the same patients (A). Killing values (mean ± SEM) from 43 independent essays are reported, results were analyzed by two way ANOVA and Bonferroni post test analysis, statistical significance is reported as * P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001; **** P ≤ 0.0001. The killing activity remained intense regardless the expression level (RFI high, intermediate, low) of CD44v6 on targets (B). Representative flow-cytometry histograms for each RFI level are reported. The addition of a blocking antibody against CD44v6 reduced the killing ability of CAR+.CIK cells to levels comparable to NTD.CIK. Blocking NKG2D receptor significantly reduced the tumor killing activity of unmodified NTD.CIK but did not impair CAR+.CIK (n = 4) (C). CAR+.CIK were moderately capable of killing monocytes at (E/T 5:1), the effect sensibly decreased and ceased at lower E:T ratios (n = 3) (D). Abbreviations: CAR Chimeric Antigen Receptor; NTD, Not Transduced; STS, Soft Tissue Sarcoma.
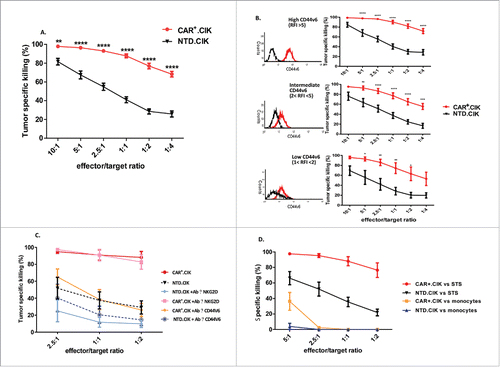
Figure 3. Cytokine production by CAR+.CIK. CAR+.CIK produced higher amounts of IL4, IFN-γ and IL6 compared with NTD.CIK. A trend toward lower production of IL5 and TGFβ was observed. Results are reported as optic density ratio (O.D. ratio) between samples and internal control (according to manufacturer instructions). Unpaired t test was adopted to compare CAR+.CIK with NTD.CIK. Significance is reported as * P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001; **** P ≤ 0.0001.
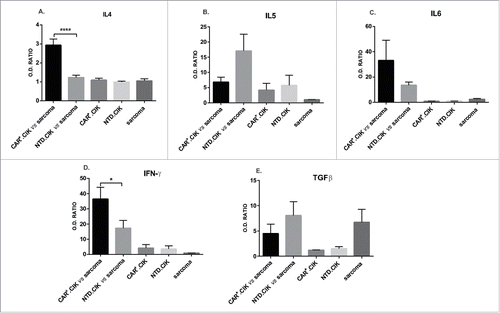
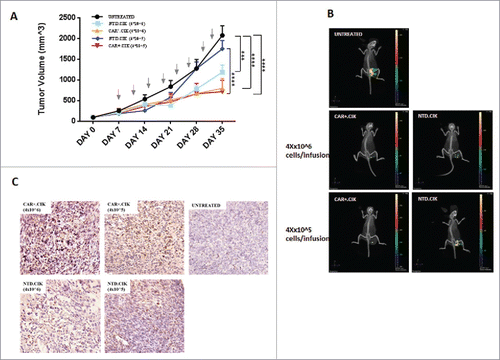
Figure 4. In vivo anti-sarcoma activity of antiCD44v6 CAR+.CIK. CAR+.CIK were intravenously infused, every 3–4 days (total 8 infusions), in NOD/SCID mice bearing subcutaneous patient-derived UPS xenograft. Two different doses (4 × 105 and 4 × 106 per infusion) of CAR+.CIK were explored. We observed a significant delay of tumor growth in mice (n = 6) treated with CAR+.CIK cells (4 × 105), compared with untreated controls (n = 6, p< 0.0001) or treated with equivalent doses of paired NTD.CIK cells (n = 6, p< 0.0001). A similar antitumor activity was observed with 1 log-higher dose of CAR+.CIK (4 × 106). Arrows indicate CIK cell infusions. Results were analyzed by Two way Anova and correction for multiple comparisons test using Bonferroni method: Statistical significance is expressed as * P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001; **** P ≤ 0.0001 (A). In selected cases (1 representative mouse per group, n = 5) results were confirmed by a 3D imaging analysis based of fluorescent glucose uptake (B). Tumor homing of CAR+.CIK was confirmed by IHC in explanted tumors by staining with anti-human CD3 antibody (C). Abbreviations: CAR Chimeric Antigen Receptor; NTD, Not Transduced; NOD SCID mice, Non obese diabetic/severe combined immunodeficiency mice.