Figures & data
Figure 1. Time course of serum anti-F protein IgG in cotton rats. Cotton rats were intranasally immunized with 3 doses of vaccine (filled arrows) followed by a live viral challenge with RSV A2 (open arrows). Serum anti-F protein IgG end point titers were determined in cotton rats vaccinated with a dose of 3.2 × 105 PFU RSV L19 (6.6 µg F protein) formulated in (A) W805EC or (B) P188. The results are presented as the geometric mean titer ± 95% confidence interval (CI) (n = 8 cotton rats per group).
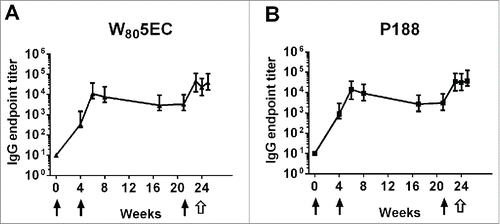
Figure 2. Immunization with RSV L19 formulated in nanoemulsion generates cross-protective neutralizing antibodies. (A) Serum neutralizing antibody titers against RSV L19 were determined at 0, 4, 5, and 21 weeks. Neutralizing titers are determined as the serum dilution which neutralizes 50% of virus. Results are presented as mean titer ± standard deviation (n = 8 cotton rats per group). Neutralizing antibody titers against both L19 and A2 virus were determined after the final immunization and prior to viral challenge in animals immunized with (B) W805EC or (C) P188. Points represent neutralization titer for individual animals. Error bars indicate standard deviation.
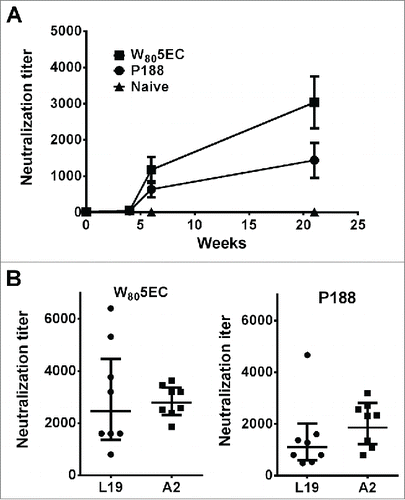
Figure 3. Intranasal vaccination with NE-RSV protects against RSV challenge. Cotton rats were vaccinated intranasally at weeks 0, 4 and 21 and challenged with 5 × 105 PFU RSV A2 at week 23. Viral clearance was assessed in lung tissue 4 d after challenge. Data are represented as PFU/g of lung tissue. The line represents the lower limit of detection of the assay.
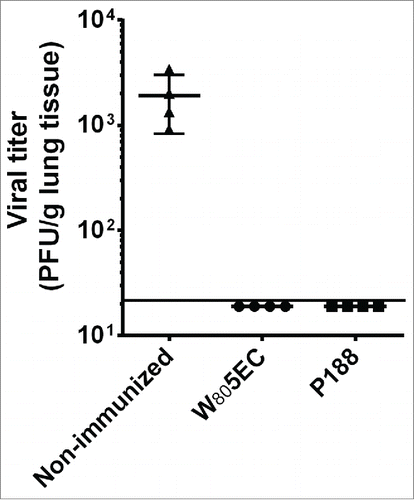
Figure 4. Splenocytes from immunized cotton rats secrete high amounts of IFN-γ. Spleens were harvested 8 d following viral challenge and were either cultured ex vivo in medium (filled symbols) or stimulated with 0.5 MOI RSV L19 (open symbols). IFN-γ in culture supernatants was determined by ELISA. Data are represented as mean ± SD. * indicates statistical difference (p<0.05).
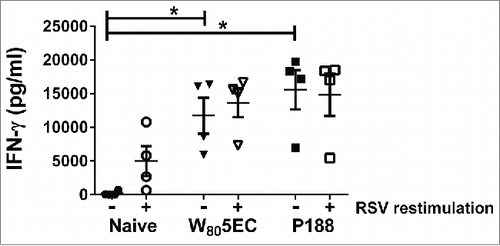
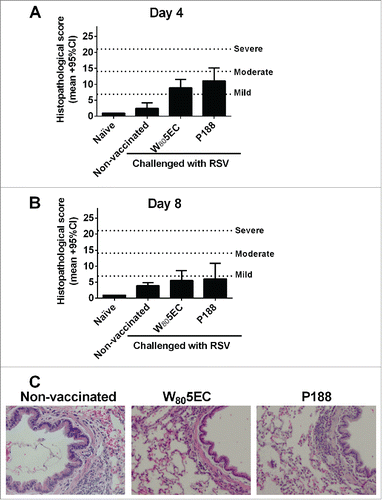
Figure 5. Evaluation of histopathological changes in the lung after immunization and viral challenge. Lungs were harvested 8 d after RSV A2 challenge and stained with hematoxylin and eosin to assess histological changes. The lung sections from days (A) 4 and (B) 8 were scored as described in the methods section, and (C) representative photomicrographs are shown. Bars represent mean ± 95% CI.