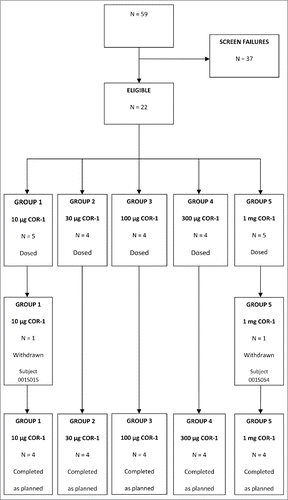
Figures & data
Table 1. Summary of demographic data.
Table 2. Study schedule of events.
Table 3. Summary of treatment emergent adverse events.
Figure 2. Injection site reactions. Scores corresponding to the injection site erythema size, measured 1 (A) and 2 (B) days after each vaccination, are plotted for subjects of cohorts that received 500 μg to 30 μg DNA vaccine. Note that the cohort which received 1 mg of DNA vaccine received 2 injections of 500 μg to both forearms. Scores of 1, 2, 3 and 4 were given for erythema measuring ∼0.5, ∼1.0, ∼1.5 and >1.5 cm, respectively.
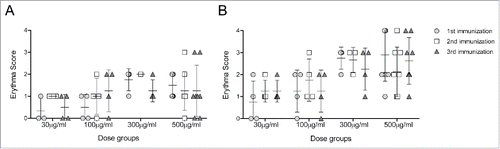
Figure 3. Pre-existing and vaccine-related cellular responses measured by IFN-γ ELISPOT. (A) Pre-existing responses to the gD2 peptide were determined using PBMCs taken at Visit 1 before the first vaccination. Responses were determined positive if X-(SD of X) – Y-(2xST of Y) > 10 (X: mean spot value; Y: mean spot value of no peptide control wells). (B) Number of subjects that were classified of having mounted a vaccine-related cellular immune response to gD2. Vaccine related positivity was accepted when the mean spot value was increased at least 2 fold compared to pre-existing responses in at least one peptide pool and at least one visit after vaccination.
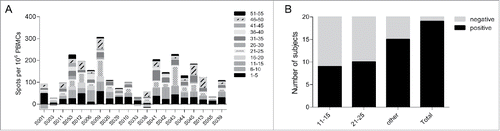
Table 4. Change in median ELISPOT count across all subjects, for each peptide pool, following immunization.