Figures & data
Figure 1. Study procedures for Study A and Study B. Notes: In Study A, all doses of PCVs were co-administered with DTPa-HBV-IPV/Hib at primary and booster vaccination (Finland, Poland and France), except for the 2nd dose in France that was co-administered with DTPa-IPV/Hib. In Study B, the PHiD-CV/MenC-CRM and PHiD-CV/MenC-TT groups received: the meningococcal vaccines as 2-dose primary vaccination in Germany and Spain, 3-dose primary vaccination in Poland (3rd dose received after blood sampling); PHiD-CV was co-administered with DTPa-HBV-IPV/Hib at primary vaccination and DTPa-HBV-IPV/Hib (Germany and Poland) or DTPa-IPV/Hib (Spain) at booster vaccination. In the PHiD-CV/HibMenC-TT and 7vCRM/HibMenC-TT groups, PCVs were co-administered with DTPa-HBV-IPV at primary vaccination and with DTPa-HBV-IPV (Germany and Poland) or DTPa-IPV (Spain) at booster vaccination. $Blood sample collected for immunologic memory assessment 7–10 days after the PHiD-CV dose at Y4, in Study A; Y = number of years following booster vaccination in PCV-vaccinated children; Pri = primary; Bst = booster; PCV = pneumococcal conjugate vaccine.
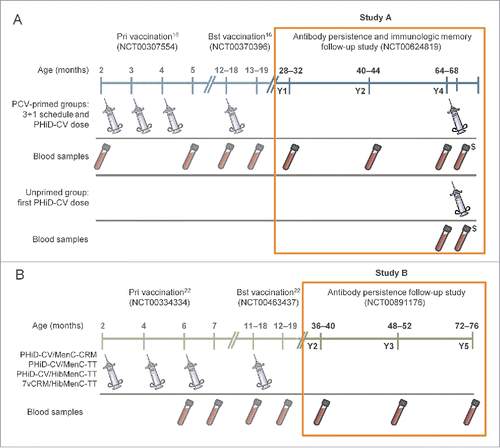
Figure 2. Flow diagram for Study A. N = number of children; Y = number of years following booster vaccination in PCV-vaccinated children; TVC = total vaccinated cohort; ATP = according-to-protocol cohort; PHiD-CV = children previously primed and boosted with PHiD-CV; 7vCRM = children previously primed and boosted with 7vCRM; 7vCRM/PHiD-CV = children previously primed with 7vCRM and boosted with PHiD-CV; Unprimed = age-matched children not previously vaccinated with any pneumococcal vaccine and enrolled as control group for the immunologic memory assessment.
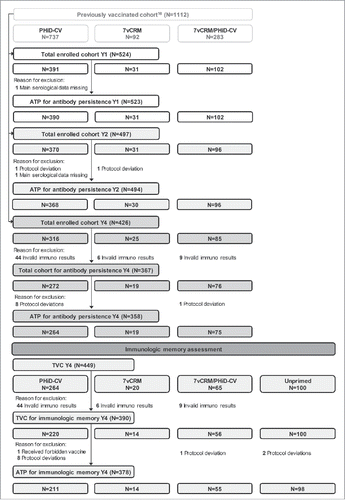
Table 1. Demographic characteristics of the study population by timepoint (ATP cohorts for the respective timepoints) (Study A and Study B).
Figure 3. Flow diagram for Study B. N = number of children; Y = number of years following booster vaccination; ATP = according-to-protocol cohort. PHiD-CV/MenC-CRM = children receiving PHiD-CV co-administered with MenC-CRM; PHiD-CV/MenC-TT = children receiving PHiD-CV co-administered with MenC-TT; PHiD-CV/HibMenC-TT = children receiving PHiD-CV co-administered with HibMenC-TT; 7vCRM/HibMenC-TT = children receiving 7vCRM co-administered with HibMenC-TT.
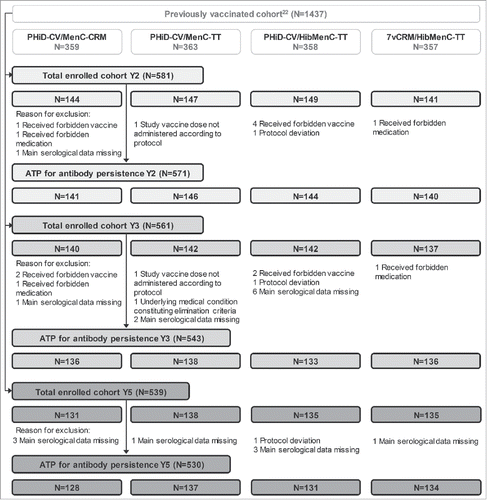
Figure 4. Serotype-specific pneumococcal antibody GMCs following primary and booster vaccination up to year 4 post-booster (Study A) (ATP cohort for immunogenicity and ATP cohorts for antibody persistence at Y1, Y2 and Y4, respectively). *vaccine-related serotypes; GMC = geometric mean concentration; ATP = according-to-protocol; Y = number of years following booster vaccination; N = maximum number of children with available results at primary vaccination/booster vaccination/Y1/Y2/Y4; pre-pri = before the 1st dose of primary vaccination; post-pri = 1 month after the 3rd dose of primary vaccination; pre-bst = before the booster dose; post-bst1 month after the booster dose; error bars indicate 95% confidence intervals.
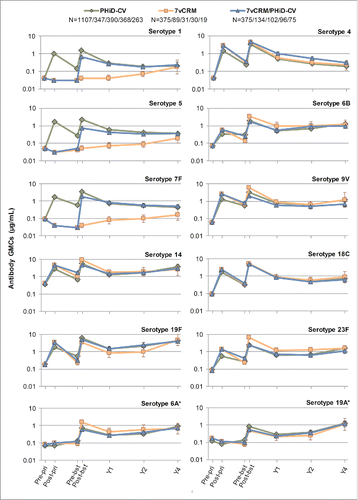
Figure 5. Serotype-specific pneumococcal OPA GMTs post-primary and post-booster vaccination up to year 4 post-booster (Study A) (ATP cohort for immunogenicity and ATP cohorts for antibody persistence at Y1, Y2 and Y4, respectively). *vaccine-related serotypes; OPA = opsonophagocytic activity; GMT = geometric mean titer; ATP = according-to-protocol; Y = number of years following booster vaccination; N = maximum number of children with available results at primary vaccination/booster vaccination/Y1/Y2/Y4; post-pri = 1 month after the 3rd dose of primary vaccination; pre-bst = before the booster dose; post-bst = 1 month after the booster dose; error bars indicate 95% confidence intervals.
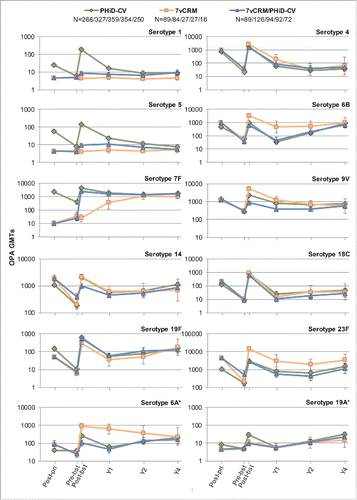
Table 2. Seropositivity rates and GMCs for anti-protein D antibodies by timepoint (ATP cohorts for the respective timepoints) (Study A).
Figure 6. Serotype-specific pneumococcal antibody GMCs post-primary and post-booster vaccination up to year 5 post-booster (Study B) (ATP cohort for immunogenicity and ATP cohorts for antibody persistence at Y2, Y3 and Y5, respectively). GMC = geometric mean concentration; ATP = according-to-protocol; Y = number of years following booster vaccination; N = maximum number of children with available results at primary vaccination/booster vaccination/Y2/Y3/Y5; post-pri = 1 month after the 3rd dose of primary vaccination; pre-bst = before the booster dose; post-bst = 1 month after the booster dose; error bars indicate 95% confidence intervals.
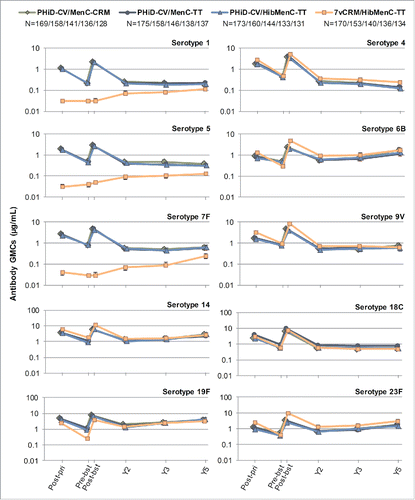
Figure 7. Serotype-specific pneumococcal OPA GMTs post-primary and post-booster vaccination up to year 3 post-booster (Study B) (ATP cohort for immunogenicity and ATP cohorts for antibody persistence at Y2 and Y3, respectively). *testing of Streptococcus pneumoniae opsonophagocytic activity (OPA) was not performed at year 5; GMT = geometric mean titer; ATP = according-to-protocol; Y = number of years following booster vaccination; N = maximum number of children with available results at primary vaccination/booster vaccination/Y2/Y3; post-pri = 1 month after the 3rd dose of primary vaccination; pre-bst = before the booster dose; post-bst = 1 month after the booster dose; error bars indicate 95% confidence intervals.
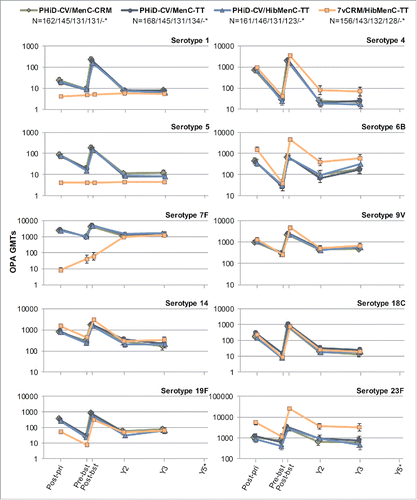
Table 3. Seropositivity rates and GMCs for anti-protein D antibodies by timepoint (ATP cohorts for the respective timepoints) (Study B).
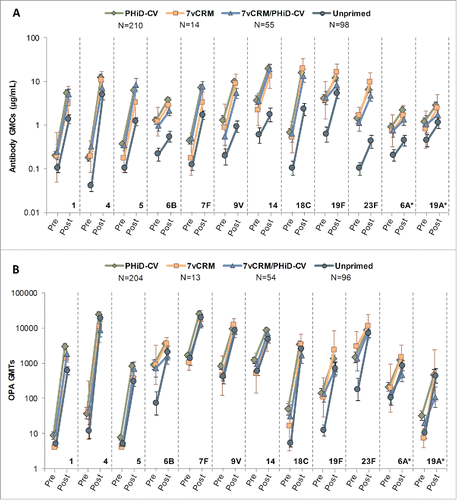
Figure 8. Serotype-specific pneumococcal antibody GMCs (A) and OPA GMTs (B) before and 7–10 days after the additional PHiD-CV dose in PCV-vaccinated children or the first PHiD-CV dose in the Unprimed group (Study A) (ATP cohort for immunologic memory at year 4). *vaccine-related serotypes; PCV = pneumococcal conjugate vaccine; ATP = according-to-protocol; GMC = geometric mean concentration; OPA = opsonophagocytic activity; GMT = geometric mean titer; N = maximum number of children with available results; pre = before the additional PHiD-CV dose in PCV-vaccinated children or before the first PHiD-CV dose in the Unprimed group; post = 7–10 days after the additional PHiD-CV dose in PCV-vaccinated children or 7–10 days after the first PHiD-CV dose in the Unprimed group; error bars indicate 95% confidence intervals.