Figures & data
Figure 1. Overview of the process required to produce a biologic medicinal product. The production process is broken down into 2 main parts. The upstream processes are responsible for genetic manipulation of a host cell and the subsequent large scale production of the drug substance. The downstream processes are responsible for the purification of the drug substance and its formulation into a drug product.
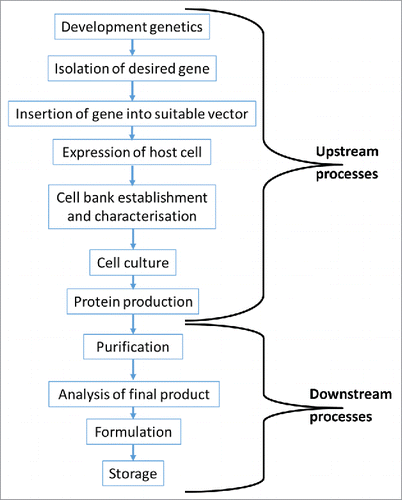
Figure 2. The development and isolation of tocilizumab producing seed cells. This figure adapted from the production section of the IV tocilizumab EPAR, details the insertion of the gene coding for tocilizumab production into a host CHO cell and the subsequent processes to isolate and culture the tocilizumab producing CHO cell into a viable master and working cell bank.
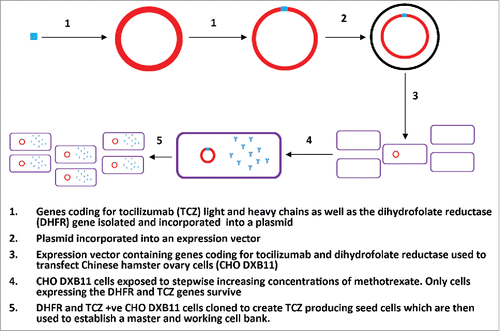
Table 1. Overview and summary of the contents of the tocilizumab European Public Assessment Report (EPAR).
Table 2. Overview of all the marketing authorisations granted for tocilizumab.
Table 3. Description and results from the pivotal clinical trials involving tocilizumab use in patients with RA.
Table 4. Description and results from the pivotal clinical trials involving tocilizumab use in patients with sJIA and pJIA.
Table 5. Description of the most common side effects associated with tocilizumab use and how to manage them.

