Figures & data
Table 1. Demographic characteristics (total vaccinated cohort).
Figure 1. Participant flow diagram.
N, number of participants in each group; TVC, total vaccinated cohort; ATP, according-to-protocol. Infants in DTPa-IPV/Hib group received 3 doses of combined diphtheria-tetanus-acellular pertussis-inactivated poliomyelitis and Haemophilus influenzae type b vaccine at 2, 4, 6 months of age and infants in DTPa-IPV+ Hib group received 3 concomitant doses of diphtheria-tetanus-acellular pertussis-inactivated poliomyelitis vaccine and Haemophilus influenzae type b vaccine at 2, 4, 6 months of age.
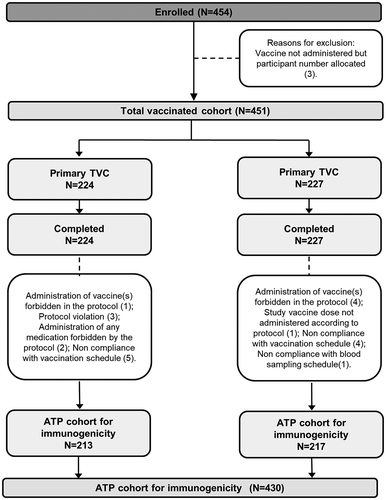
Table 2. Group differences in seroprotection/seropositivity rates and adjusted GMC ratio 1M post-dose 3 (ATP immunogenicity cohort).
Table 3. Seroprotection/seropositivity rates and GMCs/GMTs at pre-vaccination and 1M post-dose 3 (ATP immunogenicity cohort).
Table 4. Vaccine response rate to anti-PT, anti-FHA and anti-PRN antibodies 1M post-dose 3 (ATP immunogenicity cohort).
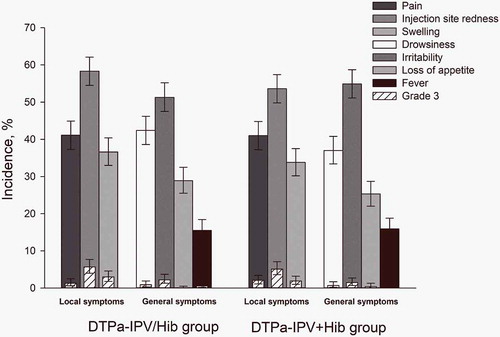
Figure 2. Incidence of solicited local and general symptoms reported up to 4 days post-vaccination (total vaccinated cohort).
Infants in DTPa-IPV/Hib group received 3 doses of combined diphtheria-tetanus-acellular pertussis-inactivated poliomyelitis and Haemophilus influenzae type b vaccine at 2, 4, 6 months of age and infants in DTPa-IPV+ Hib group received 3 concomitant doses of diphtheria-tetanus-acellular pertussis-inactivated poliomyelitis vaccine and Haemophilus influenzae type b vaccine at 2, 4, 6 months of age. The results are reported overall/dose. The error bars indicate 95% confidence intervals. Grade 3 were defined as adverse events preventing normal activity, pain upon limb movement or a spontaneously painful limb, redness and swelling >20 mm in diameter, a tympanic temperature >39.0°C, loss of appetite resulting in not eating at all, drowsiness that prevented normal activity, and irritability/fussiness resulting in crying that cannot be comforted.