Figures & data
Figure 1. Rotavirus structure and potential vaccine targets.
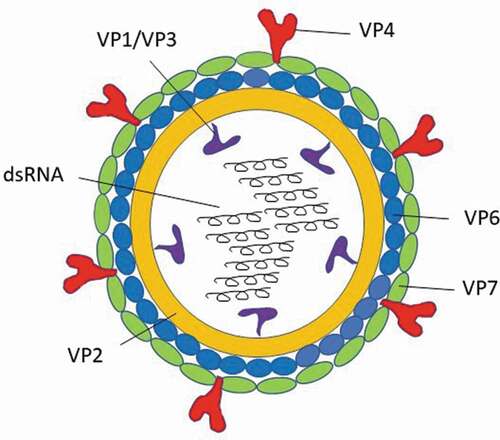
Table 1. Characteristics of rotavirus single-strain vaccines based on neonatal or live-attenuated human RV strains
Table 2. Characteristics of rotavirus single-strain vaccines based on live-attenuated animal RV strains
Table 3. Characteristics of rotavirus multi-strain vaccines based on live-attenuated bovine-human reassortant RV strains
Table 4. Characteristics of new rotavirus vaccines in early phase development
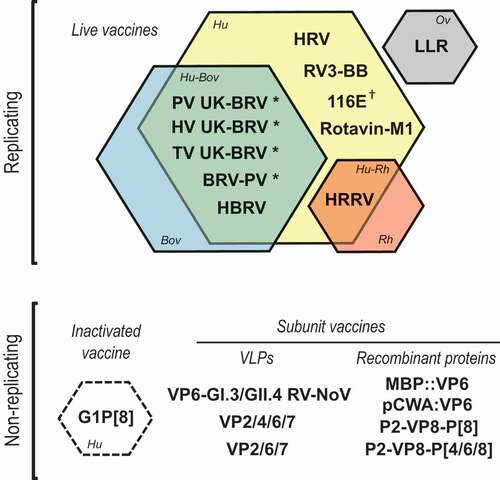
Figure 2. Overview of the rotavirus vaccines included in this review reflecting their vaccine concept and type of strain.