Figures & data
Table 1. Authorization for use in children and teens of COVID-19 vaccines validated for use by the WHO as of January 12, 2022.
Table 2. Contraindications and precautions to COVID-19 vaccination for children aged 3–17 y with underlying medical conditions.
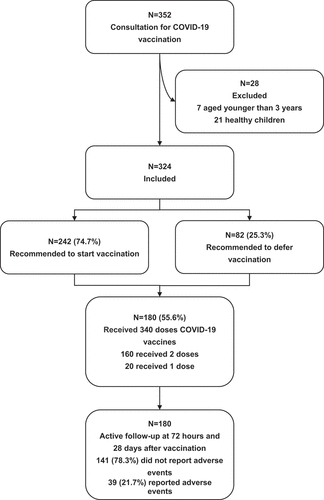
Table 3. Demographics and medical conditions between the two groups recommended to start or defer CIVID-19 vaccination in the 324 subjects.
Table 4. Adverse reactions reported within 28 d after vaccination in the 180 children receiving 340 doses of inactivated COVID-19 vaccines.
Table 5. Demographics and medical conditions between the two groups of reported adverse reactions and not reported adverse reactions within 28 d after vaccination.