Figures & data
Figure 1. Status of UPOV membership as of September 2022. Green – members of UPOV (78)(covering 97 States); Red – initiating states (19) and organisations (1); and Orange – States (23) and organisations (1) in contact with the UPOV Office.Citation61.
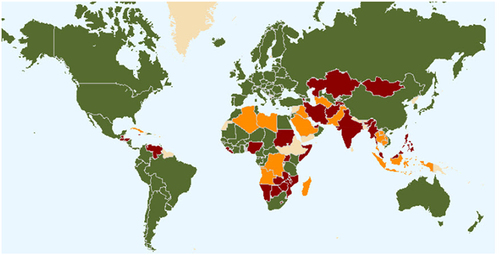
Table 1. Recent attitudes towards consumption of food generated from genetically modified and gene edited crops.
Table 2. Number of GM plant events authorised for (a) commercial cultivation and (b) for food and/or feed use - since 1992 per jurisdiction SourceCitation4,Citation174.
Table 3. Categorization of 30 countries by the regulatory system used (product versus process) and the amount of commercially cultivated GM crops. (Adapted fromCitation159).
Table 4. Difference between discounted aggregate consumer and producer surplus (US$ billion) over 40 years for four scenarios of adoption of a gene edited solution providing resistance against an emerging plant disease, Fusarium oxysporum f.Sp. cubense Tropical race 4 compared with no adoption of a solution under moderate disease incidence (adapted from.Citation248
Table 5. New Breeding Technologies (NBT) legislation for growing GM crops and forages for 56 countries plus the European Union. Refer also toCitation32, Citation34, Citation159, Citation227, Citation264, Citation288, Citation289,Citation158, Citation180, Citation228, Citation290, Citation291 andCitation292 for further detail.
