Figures & data
Figure 1 Patient disposition. *All patients randomized contributed to the primary and secondary efficacy analyses, except for 1 patient who withdrew consent right after randomization and did not receive any treatment in the placebo group; all dosed patients contributed to the safety analysis. †2 patients were off study drug too long due to prolonged hospitalization. ‡1 patient no longer wanted to participate in the study due to factors other than the study treatment or study procedures, 1 patient had difficulty traveling to clinic visits, 1 patient withdrew for personal reasons. §1 patient could not continue the study and required visits due to unforeseen work events, 1 patient withdrew due to family circumstances. ¶2 patients did not feel were benefiting from treatment and decided to discontinue, 1 patient had difficulty traveling to clinic visits. AE: adverse event; bid: twice daily; ET: early termination.
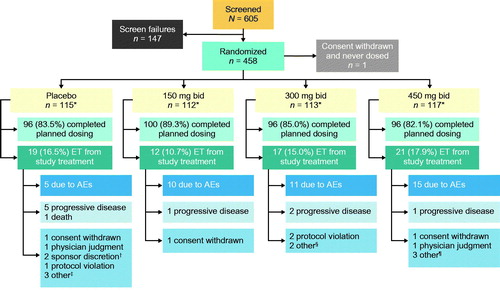
Table 1 Baseline demographics and disease characteristics.
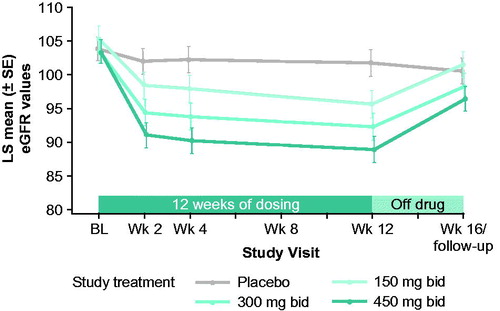
Figure 2 LS mean change from baseline for each group from a mixed model analysis of (A) percent predicted SVC (primary endpoint), (C) ALSFRS-R total score, and (E) muscle strength mega-score. Post hoc analysis of LS mean change from baseline from a mixed model analysis for all reldesemtiv-treated patients versus placebo for (B) percent predicted SVC, (D) ALSFRS-R Total Score, and (F) muscle strength mega-score. ALSFRS-R: Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised; bid, twice daily; BL: baseline; LS: least squares; SE: standard error; SVC: slow vital capacity; wk: week.
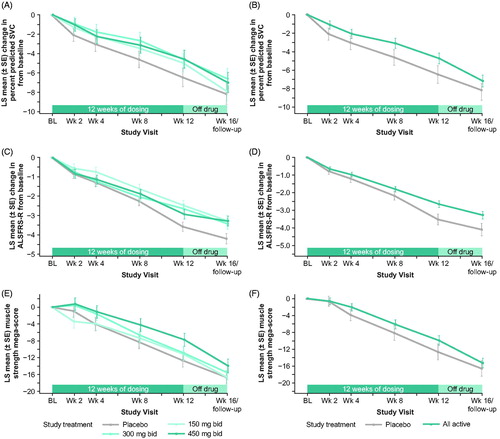
Figure 3 Forest plots from post hoc analyses of LS mean differences between treatment with reldesemtiv and placebo by subgroups for (A) percent predicted SVC, (B) ALSFRS-R, and (C) muscle strength mega-score. *Pretrial reduction of ALSFRS-R total score per month. ALSFRS-R: Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised; CI: confidence interval; LSM: least squares mean; pbo: placebo SVC: slow vital capacity.
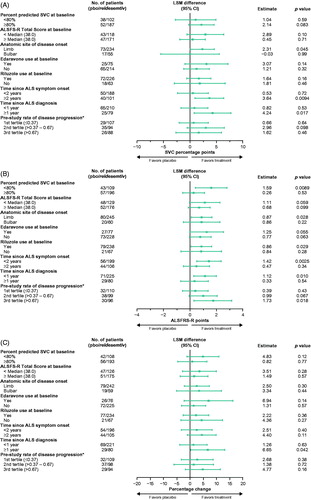
Figure 4 Post hoc analysis of change from baseline in (A) ALSFRS-R Total Score, (B) ALSFRS-R Fine Motor Domain and (C) ALSFRS-R Gross Motor Domain score by progressor tertiles. ALSFRS-R: Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised; FP: fastest progressors; LS: least squares; MP: middle progressors; SP: slowest progressors.
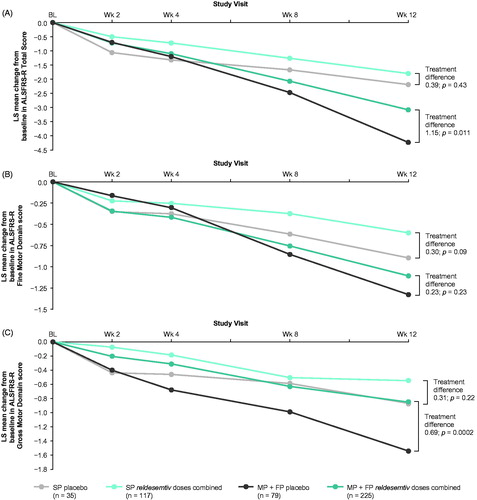
Table 2 Most common TEAEs (≥10 patients in any treatment group), serious TEAEs (>1 patient), and deaths during the trial.
Data availability statement
Data reported herein are part of a sponsor-led clinical development program that is ongoing, and thus complete datasets for the trial will not be made available with this report.