Figures & data
Figure 1 Genetic testing practices for suspected familial cases of ALS (fALS). (A) Routine genetic testing for fALS. (B) For clinics routinely ordering genetic testing for fALS, proportion of clinics ordering SOD1 sequencing and C9orf72 repeat expansion testing only, versus those ordering a broad panel of ALS genes, including SOD1 and C9orf72 (note that composition of panels vary across the laboratories used).
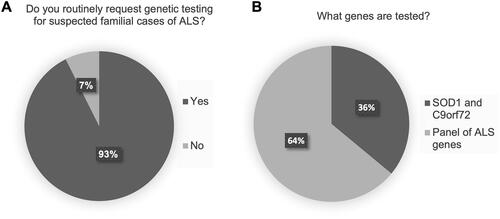
Figure 2 Genetic testing practices for seemingly sporadic cases of ALS (sALS). (A) Routine genetic testing for sALS. (B) For clinics routinely ordering genetic testing for sALS, proportion testing C9orf72 only, versus those testing for both SOD1 and C9orf72. (C) Primary barriers cited to accessing genetic testing for sALS cases.
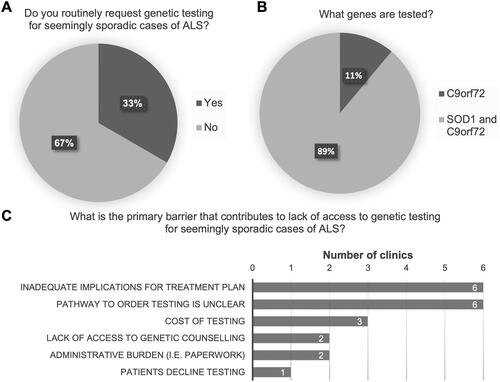
Figure 3 Predictive testing practices for at-risk, asymptomatic family members. (A) Proportion of clinics offering predictive testing to at-risk, asymptomatic family members of a symptomatic ALS patient with a confirmed genetic etiology to their ALS. (B) Circumstances under which predictive testing is offered. (C) Specialties that handle predictive testing cases. (D) Primary barriers cited to accessing genetic testing for at-risk, asymptomatic family members.
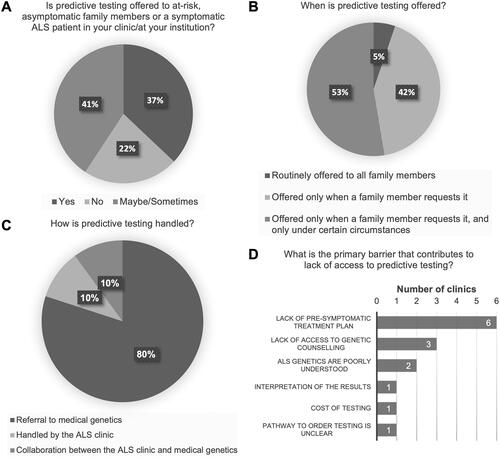
Figure 4 Clinician perspectives on genetic testing and implication for a treatment plan. Clinicians were queried as to what they deem sufficient to be considered as an implication for a treatment plan to support clinical genetic testing in the familial ALS (fALS), sporadic (sALS), and at-risk, asymptomatic family member populations. Clinicians selected only one option out of: early phase clinical trial, late-stage clinical trial, positive Phase 3 trial, and approved therapy.
Data availability statement
The data that support the findings of this study are available on request. The raw data are not publicly available to protect the anonymity of the clinics that participated.

