Figures & data
Table 1. Processes and responsibilities for the listing of medicines in government drug plans.
Table 2. 41 oncology orphan drugs by Health Canada approval date.
Table 3. 41 non-oncology orphan drugs by Health Canada approval date.
Table 4. Health Canada, FDA and EMA regulatory review times.
Table 5. Flow of the 82 orphan drugs through CADTH and the pCPA.
Table 6. Time for CADTH and pCPA processes & between approval and pCPA completion.
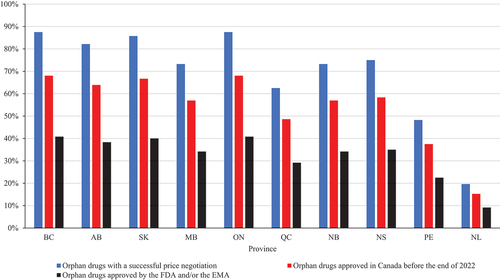
Figure 1. Listing rates for orphan drugs with successful price negotiations in Canada, approved in Canada, and approved by the FDA and/or the EMA.
Data availability statement
The data used in this analysis are available from publicly accessible resources [Citation8–23, Citation25–31,Citation53–56].

