Figures & data
Figure 1. Number of oncologic orphan drug designations (n=2,355) and approvals (n=317) from 1983–2022.
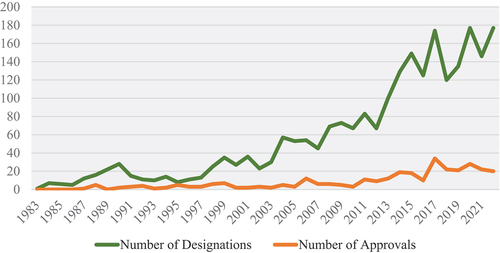
Table 1. Top 10 rare cancers with the most orphan drug designations (1983–2022).
Table 2. Top 10 orphan drug-designated cancers with the most initial approvals (1983–2022).
Figure 2. Oncologic orphan drug designations (and approvals) by organ system, 1983–2022.*Miscellaneous category includes tissue agnostic/biomarker-defined cancers, and genetic cancer syndromes
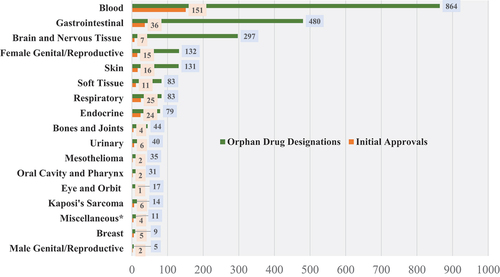
Table 3. Classification of oncologic orphan drug designations (and Approvals) by site group and site, 1983—2022 (Designations, n = 2,355) (Approvals, n = 317).

