Figures & data
Figure 1. Small GTPases in endothelial barrier regulation. Schematic, as seen from above, represents disorganized junctional complex versus organized junctional complex. Solid arrows depict pathway activation and dotted arrows indicate inactive pathway. (A) Pro-inflammatory cytokines or growth factors bind and activate their corresponding GPCRs or RTKs and activate the RhoA/ROCK/pMLC signaling pathway, which promotes formation of stress fibers and disrupts junctions causing an increase in paracellular permeability. (B) Activation of EPAC via cAMP or cAMP analog activates Rap by promoting GTP exchange. Rasip1 localizes to the junctional complex by binging HEG1 and interacting with active Rap at the cell border. Rasip1 can dimerize via its RA domain and bind 2 Rap molecules at the plasma membrane. The active Rap-rasip1 complex and radil activates ArhGAP29, which inhibits RhoA activity and prevents loss of barrier.
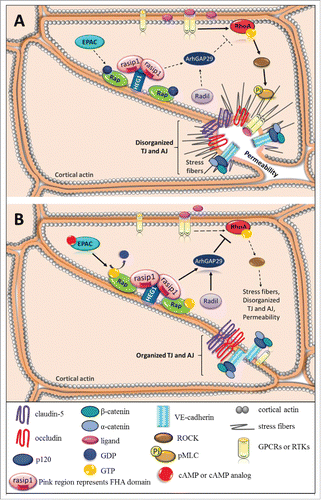
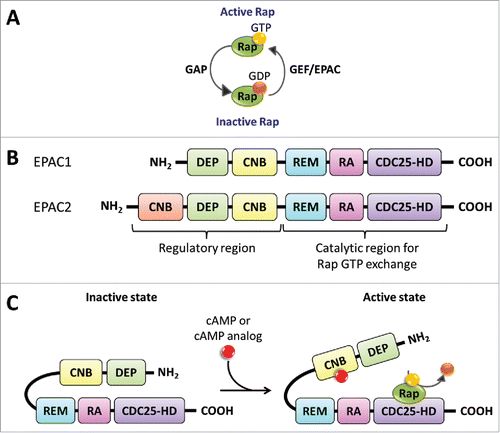
Figure 2. EPAC structure and Activation via cAMP. (A) Rap proteins are GTPases that hydrolyze GTP into GDP. GAPs increase the rate of GTP hydrolysis leading to GDP bound Rap, the inactive state. On the other hand, GEFs, such as EPAC, facilitate the exchange of GDP for GTP promoting the GTP bound Rap, the active state. (B) EPAC has 2 known isoform proteins, EPAC1 and EPAC2, encoded in separate human genes, RAPGEF3 on chromosome 12 and RAPGEF4 on chromosome 2, respectively. EPAC's catalytic region contains the CDC25-HD, RA, and REM domains. The regulatory region contains the CNB and DEP domains. The EPAC2 protein contains an additional CNB region. (C) EPAC exists in an autoinhibitory inactive state with the regulatory region suppressing the guanine nucleotide exchange (GEF) function of the catalytic region. The binding of cAMP to the CNB domain, located in the regulatory region, promotes a conformational change from an inactive to an activate state. The active EPAC binds Rap GTPases and facilitates the exchange between GDP to GTP.