Figures & data
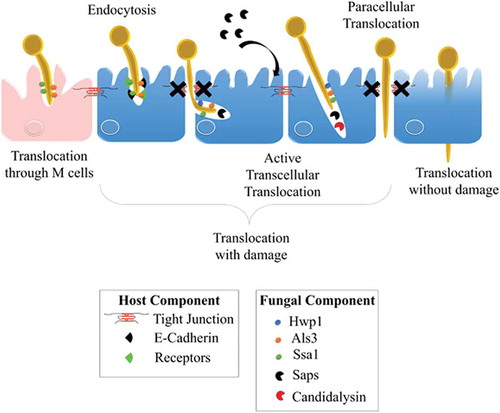
Figure 1. The intercellular junctions between enterocytes at the digestive barrier.
(A) Composition and organization of the enterocyte-enterocyte junctions in the intestinal epithelium. Tight junctions (or TJs) are the first intercellular junctions present at the apico-lateral region of enterocytes followed by the adherens junctions (AJs), the desmosomes and finally the GAP junctions at the baso-lateral region. (B) Composition and organization of the TJs and AJs. The TJs and the AJs form circumferential junctions composed by transmembranous proteins, including Claudins, TJ associated Marvel domain containing Occludin, Tricellulin and Marvel D3, and JAMs for TJs and E-cadherin and Nectin for AJs, all connected to the cytoskeleton through various proteins (i.e. Zonula Occludens (ZO1-3) proteins, Cinguline, Catenin and Afadin).
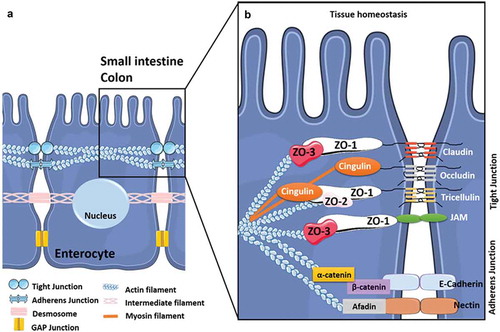
Figure 2. Organization of the gastro-intestinal tract.
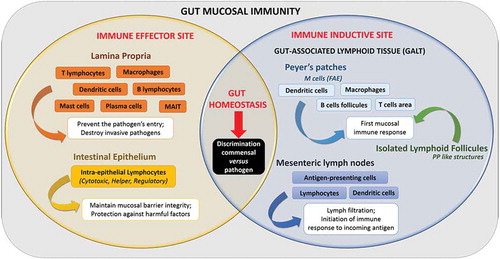
The gastro-intestinal tract forms a physical and biochemical barrier capable of segregating microorganisms from the host with the ability to discriminate commensals from pathogenic microorganisms. The physical barrier consists of a monolayer of cells, which includes various intestinal epithelial cell types (IECs) differently organized from the small intestine to the colon. The biochemical barrier consists of a mucus layer whose composition, structure and also properties differ between the small intestine and the colon. In the small intestine, the mucus is composed of a highly dynamic monolayer, not anchored to the surface of the epithelial cells. This monolayer of mucus is permeable to the bacteria. However, the distal peristaltic movements keep the microorganisms away from the surface of the epithelial cells. In the colon, the mucus is organized in two layers: the inner layer and the outer layer. The inner layer is in perpetual renewal (approximately every 1 to 2 hours) in order to remain totally germ-free. This layer is firmly anchored to the epithelial barrier through the interaction between mucins in the mucus and the mucins-binding protein located at the surface of the epithelial cells. Due to its size-exclusion filter function (i.e. exclusion of any element of more than 0.5 μm), the inner layer of mucus is impermeable to microorganisms. Finally, the outer layer is the normal habitat of intestinal commensal microbiota. In addition to the secretion of bioactive molecules such as nutrients, hormones, neuropeptides, cytokines and lipids in the gut lumen, the gut microbiota takes an active part in this permanent remodeling as highlighted in germ-free animals in which a thinner layer of mucus is observed as the result of a decreased number of goblet cells compared with conventional animals.Citation61–Citation63 The outer-most layer of mucus is a reservoir of dense populations of commensal microorganisms whose composition is linked to the existing luminal populations.Citation64 Consequently, normal or altered GM and mycobiota will influence goblet cell function as well as the composition and volume of the mucus layer by mechanisms probably involving both the direct effect of locally released microbial factors and/or the indirect effect of bioactive or immune factors resulting from the host-response to intestinal microbes. Moreover, the mucus layer is also a biochemical barrier thanks to the presence of various secreted antimicrobial factors mostly secreted by the cytoplasmic granule-rich Paneth cells (PCs). These PCs are located at the base of the small intestinal crypt in healthy individuals.Citation65 Various factors, including cholinergic agonists, bacteria and bacterial products (such as lipopolysaccharides and lipoteichoic acid),Citation66,Citation67 lead to the discharge of PC granules from the crypt into the mucus layer, forming a biochemical barrier that is crucial for establishing baseline homeostasis for mucosal and systemic inflammatory response. The PCs mainly secrete a panel of antimicrobial peptides (AMPs), which are released in intestinal mucus layer in the small intestine in humans. The composition of the mucus changes along the GI tract, contributing to the increase of the amount of microorganisms in the digestive microbiota between the small intestine and the colon.
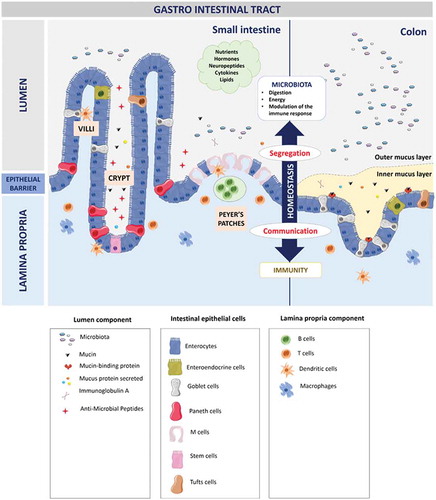
Figure 4. Invasion of C. albicans through the intestinal epithelial barrier.
Schematic representation of the different mechanisms used by C. albicans to translocate through the gut mucosa: (i) the transcellular route, (ii) the paracellular route, (iii) the translocation through M cells, and (iv) the alternate route that may occur. Als3, Agglutinin-Like Sequence 3; Ssa1, Heat shock protein ssa1; Hwp1, Hyphal wall protein 1; Saps, Secreted Aspartyl Proteases; CL, Candidalysin; TJs, Tight Junctions; LDH, Lactate dehydrogenase; Ca2+, Calcium.
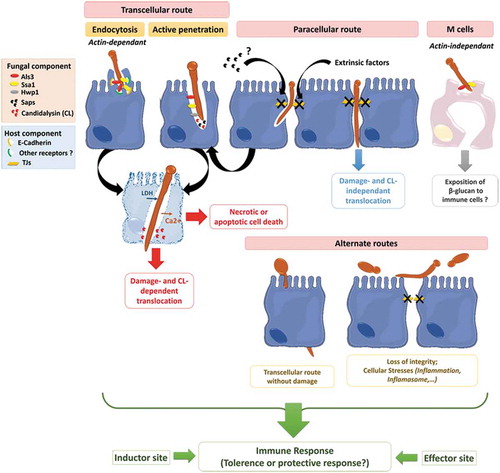
Table 1. In vitro models developed to study the interactions between Candida albicans and the epithelial intestinal barrier.
Table 2. In vivo models described to study the interaction between Candida albicans and the epithelial intestinal barrier Murine models of intravenous disseminated candidiasis.