Figures & data
Figure 1. Schematic illustration of the epithelial junctional complexes.
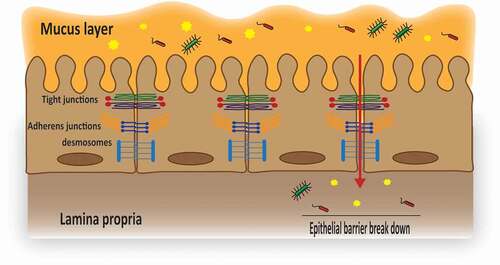
Figure 2. Schematic representation of the molecular structure of different transmembrane tight junction (TJ) proteins and the related direct interaction with different transmembrane as well as cytoplasmic scaffolding proteins. OCLN, CLDNs and tricellulin have a similar topography, with one intracellular, two extracellular loops, four transmembrane domains, and cytoplasmic N- and C-terminal domains. JAMs are characterized by two extracellular Ig-like domains, a transmembrane domain and a C-terminal cytoplasmic domain. The C-terminal cytoplasmic domain has been shown to be crucial in membrane targeting of OCLN, CLDNs and JAMs to the TJ network, whereas, both N- and C-terminal domains of tricellulin seem to play a relevant role in tricellulin localization at the TJ network. Homophilic interactions of different transmembrane TJ proteins from adjacent cells (between proteins of the same kind) are mediated through the two extracellular loops (OCLN and tricellulin), a second extracellular loop (CLDNs) or membrane-distal extracellular Ig-like domains (JAMs). The C-terminal domain of OCLN, CLDNs and JAMs interacts with different cytoplasmic scaffolding TJ proteins, including ZOs and cingulin, by which they are connected to the actin cytoskeleton.
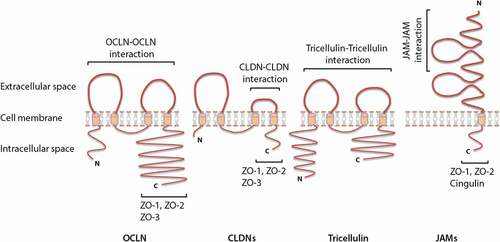
Figure 3. Immunofluorescence picture Magnification 400X (a) and schematic drawing (b) of the bicellular junctions (contact sites between two adjacent cells) and tricellular junctions (contact sites between three adjacent cells). The insert is a (non-defined) enlargement of the area marked in the original picture to visualize the bicellular junctions in more detail.
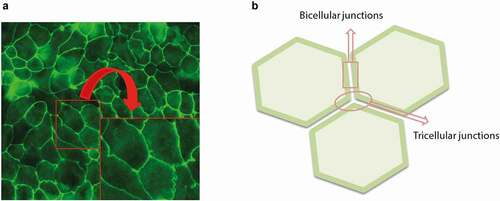
Figure 4. Schematic diagrams of the structural organization of individual cytoplasmic scaffolding proteins and the related direct interaction with different tight junctions (TJs) as well as adherens junctions. ZO proteins carry three PDZ domains, a Src homology 3 (SH3) domain, a guanylate-kinase homology (GUK) domain and proline-rich (PR) region. Afadin consists of two Ras-binding domains (RA1 and RA2), a forkhead-associated (FHA) domain, a diluted (DIL) domain, a PDZ domain and three proline-rich (PR) domains. Cingulin exists as a parallel homodimer of two subunits, each comprised of a N-terminal globular head region, a long α-helical coiled-coin rod region and a small globular tail.
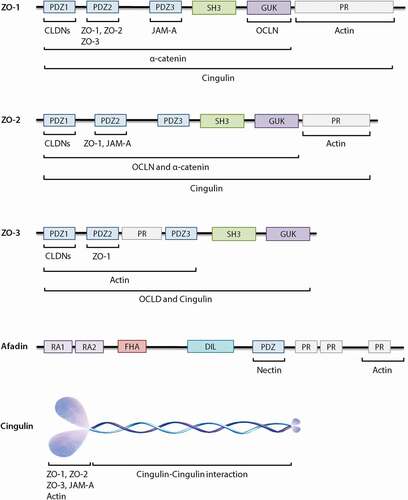
Table 1. The expression pattern of different CLDNs along the mouse intestineCitation24,Citation30,Citation58,Citation59.

