Figures & data
Figure 1. Schematic representation of HIV electrochemical detection on chitosan/Fe3O4 SPE, using MB as an intercalator (Reuse with permission from Elsevier) (Dai Tran et al. Citation2011).
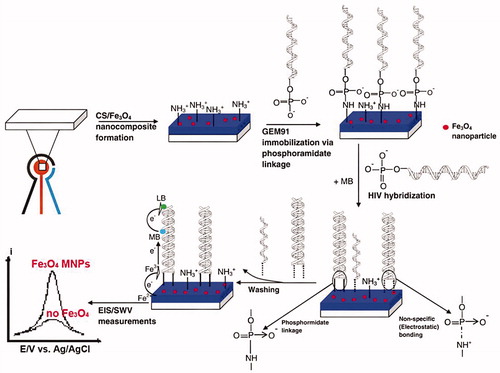
Figure 2. Quantitative detection of different target DNA concentrations by SWV is shown (Reuse with permission from Elsevier) (Dai Tran et al. Citation2011).
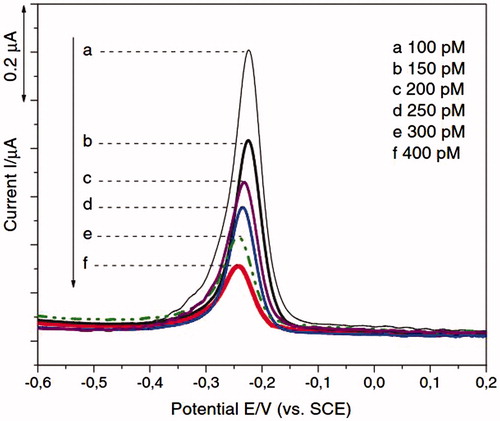
Figure 3. (Left) Cyclic voltammogram of different concentration of HIV-1 VLPs after immobilization of fragmented antibody (a) 600 fg/mL, (b) 3 pg/mL, (c) 15 pg/mL, (d) 75 pg/mL, and (e) 375 pg/m, respectively and (Right) linear plot of anodic current peak as a function of HIV-1 VLPs range from 600 fg/mL to 375 pg/mL (R = 0.958) (Reuse with permission from Elsevier) (Lee et al. Citation2013).
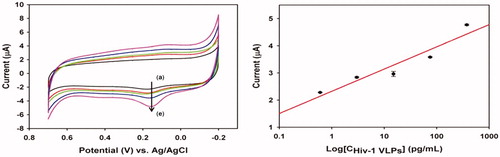
Figure 4. Impedimetric responses of AuNRs sensor with deferiprone and without deferiprone were shown (Reuse with permission from Elsevier) (Narang et al. Citation2015).
Figure 5. (A) Schematic representation of the enzyme-free and label-free ultrasensitive electrochemical DNA biosensor based on long-range self-assembled DNA nanostructures. (B) DPV responses of the gold electrode modified with various oligonucleotides: (a) CP, (b) CP + TD, (c) CP + AP1 + AP2, (d) CP + TD + AP1, and (e) CP + TD + AP1 + AP2. The concentration of TD is 10 pM. The concentrations of AP1 and AP2 are both 1 μM (Reprinted with permission from American Chemical Society) (Chen et al. Citation2012).
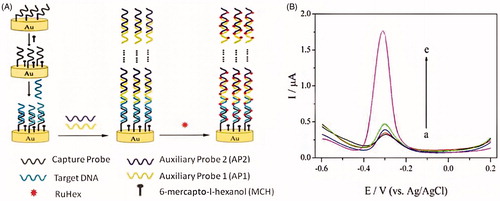
Figure 6. A layer of PPy film was electrodeposited on the platinum electrode surface, and then the Au–Ag nanocomposite was bonded directly onto the surface of PPy, which is a layer of polycation. Finally, mercapto oligonucleotide was self-assembled onto the Au–Ag nanocomposite surface.
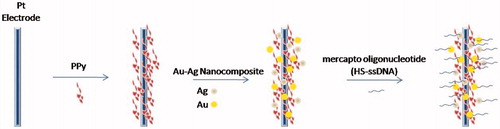
Figure 7. Schematic representation of preparation of immunosensor array and detection strategy by sandwich-type immunoassay (electrochemical voltammetry analysis) is shown. firstly, the immunosensors were fabricated by layer-by-layer coating MWCNTs, Si-HRP, Chitosan, glutaraldehyde composite on the working electrode; after that, the immunosensors were incubated with primary antibody 1 (Ab1); secondly, the primary Ab1 on the immunosensors were biorecognition with the corresponding antigens Ag; and thirdly, the immunocomplex-coated Ag were reacted with second antibodies labeled with TH/GO (Reprinted with permission from American Chemical Society) (Fang et al. Citation2015).
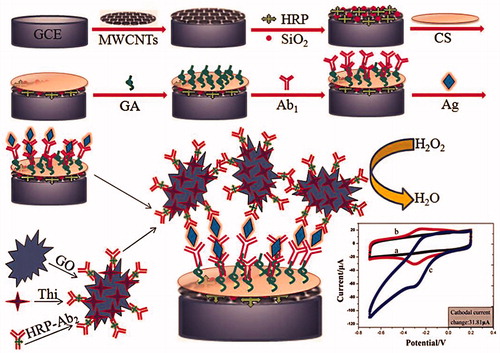
Figure 8. DPV of SWCNT/AuNP modified gold electrodes modified with Fc-pepstatin conjugate in the presence of different concentrations of HIV-1 PR at: (a) 0 pM, (b) 5 pM, (c) 10 pM, (d) 100 pM, and (e) 1000 pM. Ag/AgCl was used as the reference electrode at 100 mV/s. The assay buffer consisted of 0.1 M sodium acetate, 2 M NaClO4, 1 mM EDTA, 1 mM DTT, 10% DMSO, pH 7.4. The E of the Fc/Fc+ couple under the experimental conditions is 448 (5) mV (Reprinted with permission from Taylor & Francis) (Mahmoud and Luong Citation2010).
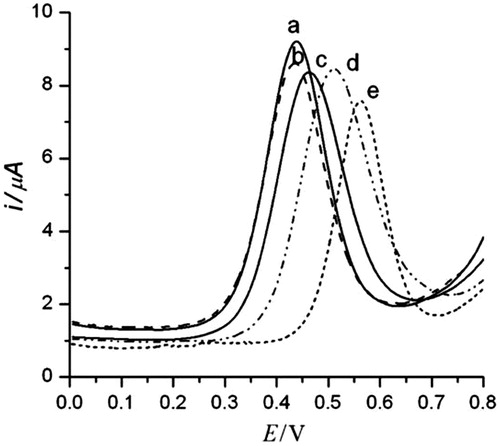
Figure 9. Schematic illustration protocol of the preparation of ferrocene- pepstatin conjugate/thiolated SWCNT and AuNP modified electrodes and their use for detecting HIV-1 protease and the subsequent assay of HIV-1 protease inhibitors. (Reproduced by permission of The American Chemical Society) (Mahmoud and Luong Citation2008).
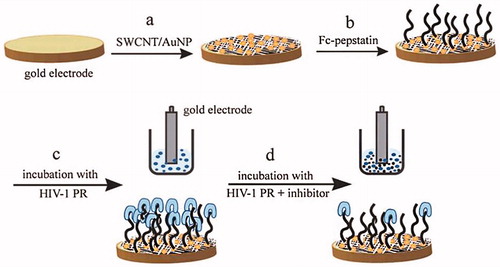
Figure 10. Demonstrated of the fabrication and detection procedure of the immunosensor (by permission from the authors (Gan et al. Citation2013)).
Figure 11. Schematic illustration of principle of immunosensor using VSNEA. (Left) The VSNEA fabricated and coated by Au. (Center) Artificial peptide sequences attached on Au-coated VSNEA by covalent interaction. (Right) RNAs attached to peptides and current path is blocked reducing Fe(CN)63-/4- redox reaction (Lee et al. Citation2016).
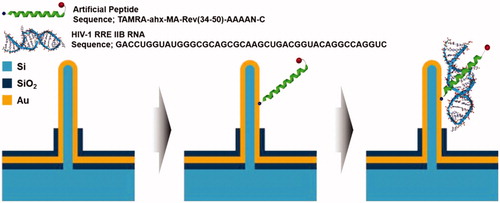
Table 1. Some of the studies listed based on electrochemical detection methods.

