Figures & data
Figure 1. Characterization of the bHb samples. Size exclusion chromatography analysis (a) of the bHb samples was carried out on a Superdex 200 column at room temperature. SDS-PAGE analysis (b) was carried out on a 12% Tris-glycine gel. Lane 1, molecular standards; Lane 2, bHb; Lane 3, Pr-bHb; Lane 4, dex40-bHb; Lane 5, dex20-bHb. The thiol groups of the bHb samples (c) were measured by titration with 4-PDS. Dex20-bHb-1 and dex40-bHb-1 were dex20-bHb and dex40-bHb without protection of Cys-93(β), respectively. Dynamic light scattering analysis (d) of the bHb samples was carried out by a DynaPro Titan TC instrument.
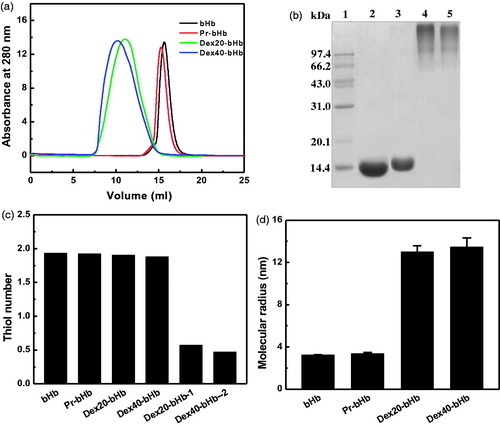
Figure 2. Structural characterization of the bHb samples. High frequency resonance Raman spectra of the bHb samples (a) were measured with exposure times of 180 s. Circular dichroism spectra of the bHb samples (b) were recorded at room temperature in the far-UV regions of 360–480 nm.
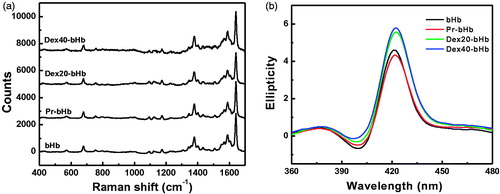
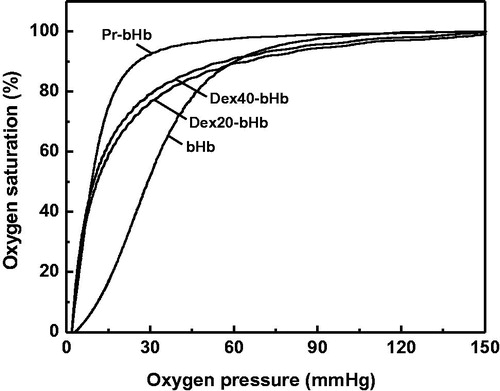
Figure 3. Tetramer stability of the bHb samples in the presence of 0.9 M MgCl2. bHb (1), Pr-bHb (3), dex20-bHb (5) and dex40-bHb (7) were loaded on a Superdex 200 column at room temperature, using PBS buffer containing 0.9 M MgCl2 (pH 7.4) as the eluent. bHb (2), Pr-bHb (4), dex20-bHb (6) and dex40-bHb (8) were loaded on a Superdex 200 column at room temperature, using PBS buffer (pH 7.4) as the eluent.
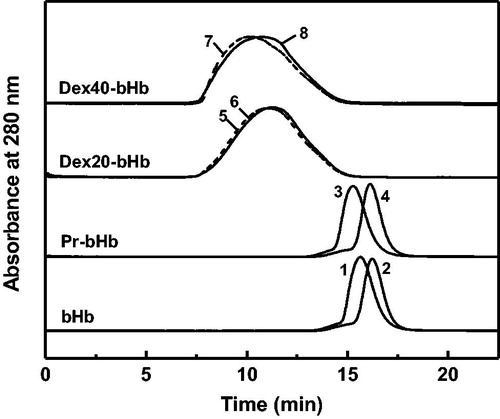
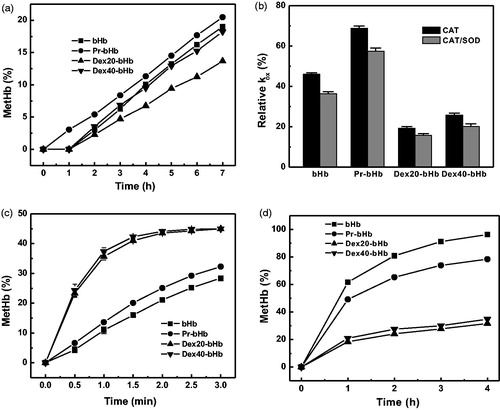
Figure 4. The autoxidation rates of the bHb samples. The autoxidation rates of the bHb samples in the presence of PBS buffer (a), the antioxidant enzymes (b), 0.1 M azide (c), and 0.9 M MgCl2 (d) were measured as a function of incubation time at 37 °C.