Figures & data
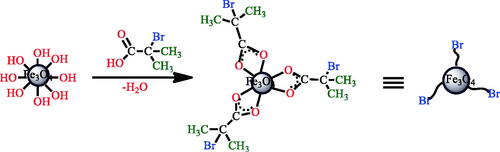
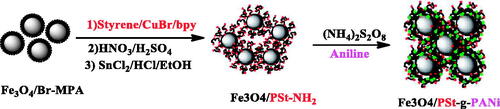
Scheme 1. Strategies for the preparation of well-defined and conductive polymer-Fe3O4 nanocomposites.
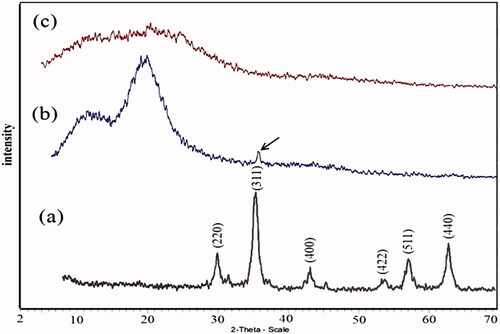
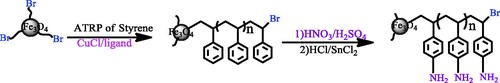
Scheme 3. Metal catalyzed controlled radical polymerization of styrene onto Fe3O4 nanoparticle and functionalization of grafted polymer.
Scheme 4. Synthesis of conductive copolymer nanocomposite by in situ chemical oxidating polymerization.
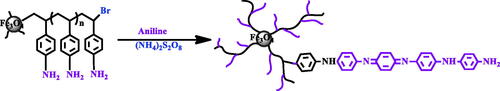
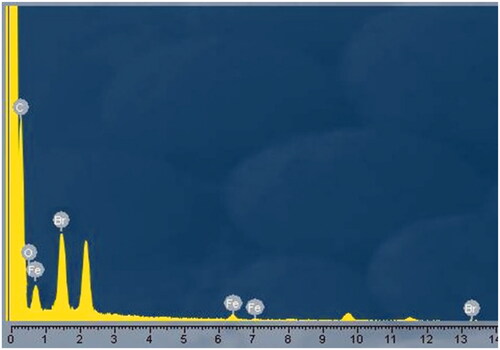
Table 1. Energy-dispersive X-ray (EDX) analysis of Fe3O4-Br-MPA macroinitiator.
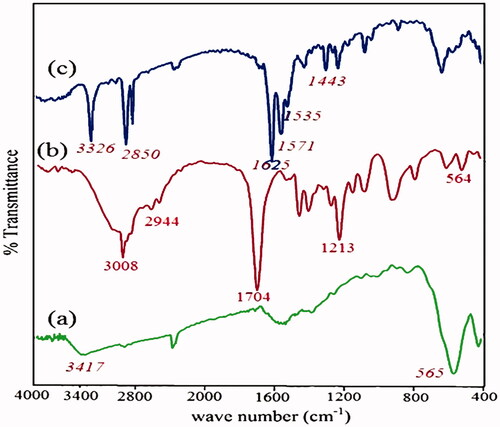
Figure 8. Thermogravimetric analysis of (a) Fe3O4 nanoparticles, (b) Fe3O4/PSt, (c) Fe3O4/PSt-g-PANi nanocomposite.
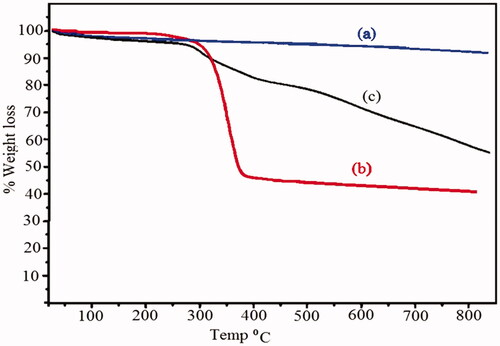
Figure 9. Transmission electron microscopy (TEM) images of (a) Fe3O4 nanoparticles (b) Fe3O4/PSt-g-PANi nanocomposite.
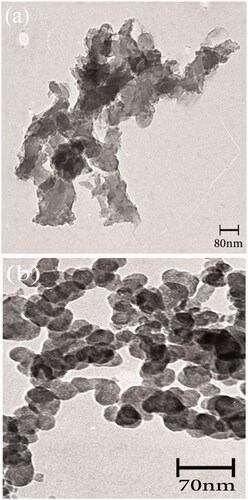
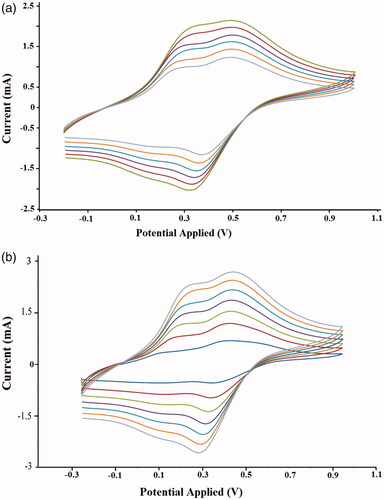
Table 2. Characteristics of the conductive nanocomposite.