Figures & data
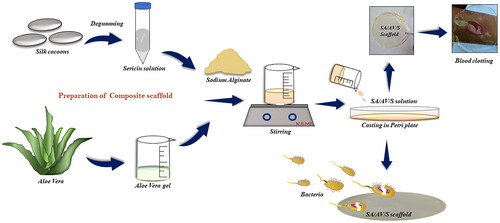
Figure 1. Schematic representation showing the various steps involved in the fabrication of SA/AV/S scaffold.
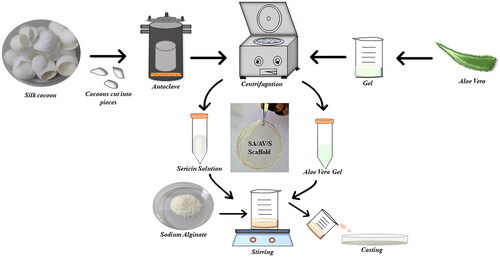
Figure 3. UV–visible absorption spectra of (A) sericin (S), (B) aloe vera (AV), (C) sodium alginate (SA) and (D) SA/AV/S scaffold.
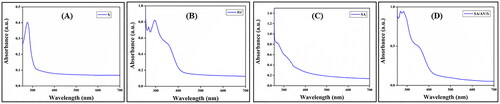
Figure 5. (A) Swelling ratio of SA/AV/S scaffold in PBS at different time intervals. (B) Haemolytic assay for SA/AV/S scaffold performed using human erythrocytes. Water and PBS treated with RBCs were used as positive and negative control, respectively.
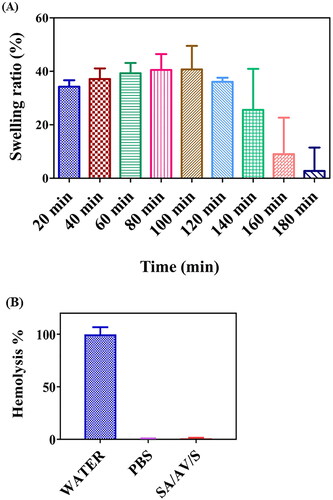
Figure 6. ( A) In vitro whole blood clotting assay for SA, AV, S and SA/AV/S scaffold, respectively. (B) Blood absorption assay for SA/AV/S scaffold. (C) Percentage of blood absorption for SA, AV, S and SA/AV/S scaffold, respectively.
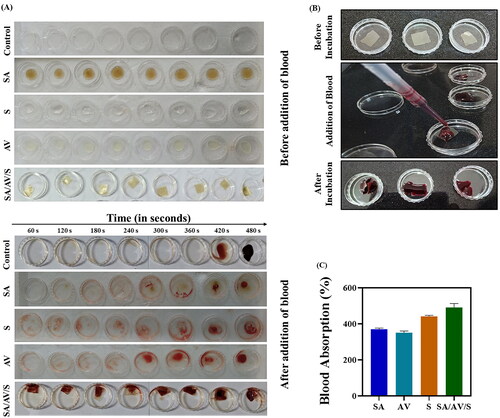
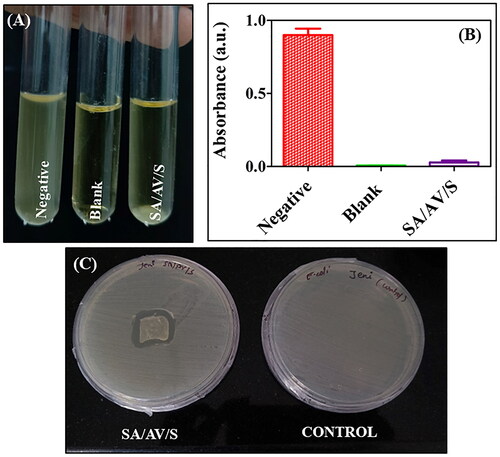
Figure 7. ( A) Turbidity analysis showing the images of tubes containing negative control, blank and E. coli strain incubated with SA/AV/S scaffold. (B) Turbidity analysis showing the optical density of negative, blank and SA/AV/S scaffold. (C) Zone of inhibition formed after treating with SA/AV/S scaffold.
Data availability statement
The authors state that all data related to this study are available within the article.