Figures & data
Figure 1. Light micrograph of microcrystalline (MC) and TEM micrograph of nanocrystalline (NC) biomedical stainless steels (adapted from references 1–3).
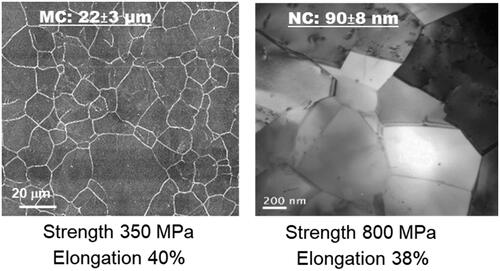
Figure 2. Fluorescence microscopy of fibroblasts nuclei with Hoechst 33258 after 24 h culture on: (a) MC and (b) NC surfaces. NC surface shows higher cell density (b) with abundant extracellular matrix formation as compared to MC (a) surface. (c) Histograms showing higher initial cell density and viability of fibroblasts on NC and MC surfaces using MTT assay (adapted from references 3, 37, 40).
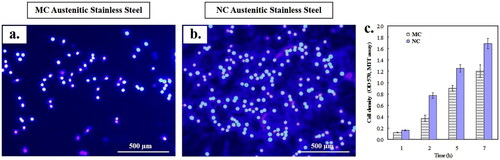
Figure 3. Fibroblasts attachment morphology at 1 h on NC (a, b) and MC austenitic stainless steel (c, d). At low magnification, cells on NC exhibited greater number of attached cells and numerous cellular extensions with thicker extracellular matrices compared to the CG surface. Cells on CG surface is spherical. High magnification micrographs (b, d), cell show higher extent of spreading on NC surface than on MC austenitic stainless steel. Cells on NC austenitic stainless steel show numerous cellular extensions are indicative of extensive attachment and interaction with the substrate (adapted from references 37, 40).
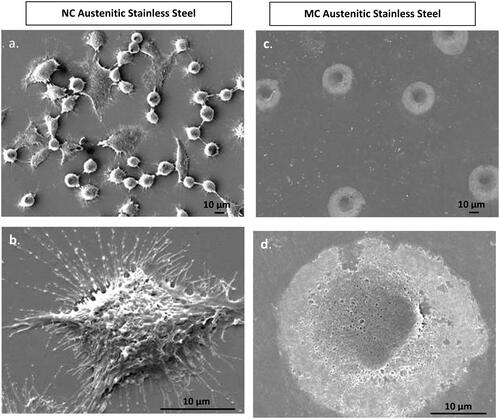
Figure 4. Scanning electron micrographs of fibroblasts morphology at 24 h on NC (a, b) and MC austenitic stainless steel (c, d). Fibroblasts grown on NG/UFG and CG surfaces are significantly different. After 24 h culture, fibroblasts on NC surface exhibit more extensive spreading, interconnectivity and thicker extracellular matrices as compared to MC surface (adapted from references 37, 40).
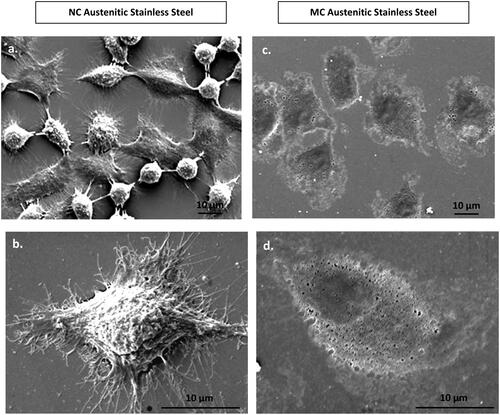
Figure 5. Fluorescence micrographs representing immunocytochemistry of fibronectin expressed by fibroblasts after incubation for 2 days on (a) MC and (b) NC stainless steel surface. A higher fluorescence intensity and expanded network of fibronectin expression along with a higher cell density is observed labelling of cell nuclei with DAPI. Inset is the magnified view of the cell (adapted from references 3, 37, 40).
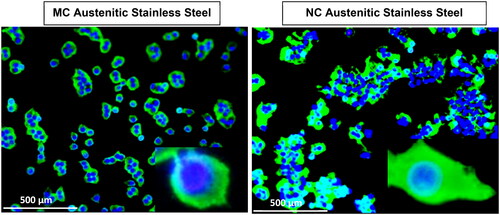
Figure 6. Focal contacts and actin stress fibres of fibroblasts after 2 days culture on MC (a,b) and NC (c,d) stainless steel surfaces. Vinculin (a, c) staining shows a larger number of focal contact sites in fibroblasts grown on the surface of NC(c) compared to cells grown on MC surface (a). The higher number of focal adhesion points corresponded well with a higher number of actin stress fibres on NC surface (d) compared to MC surface (b) (adapted from references 3, 37, 40).
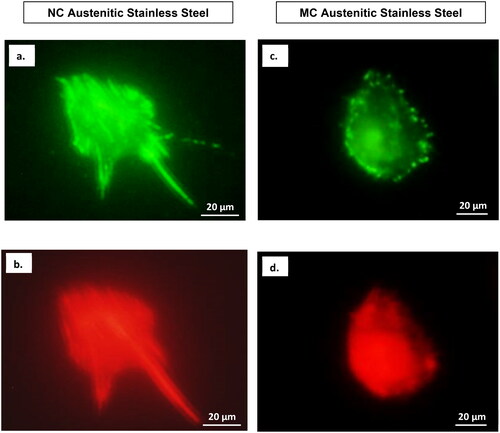
Figure 7. Confocal micrographs show combined distribution of cytoskeletal organisation and focal adhesion contacts. Actin (red), vinculin (green) and nucleus (blue) in fibroblasts cultured for 2 days on NC (a) and MC (b) stainless steel surfaces. The focal contact sites where vinculin is linked at the actin leading edges, which connect cells to the steel surface (adapted from references 37, 40).
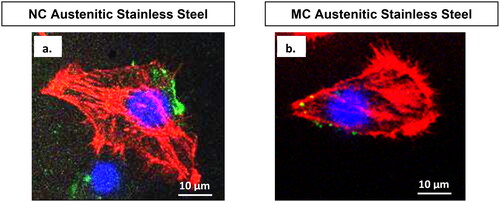
Table 1. The significance and global contribution of crystal boundaries, bio-physical characteristics, and average crystal boundary length/cell for nanocrystalline and microcrystalline surfaces.
Data availability
Given that it is an overview article with a perspective, data availability is not applicable. The authors have done their best in citing the relevant articles.

