Figures & data
Figure 1. Study design.
Abbreviations: COVID-19 = Coronavirus Disease, CP = convalescent plasma, AE = adverse event, SAE = Serious adverse event.
1One of these participants was administered corticosteroids before the study drug infusion but was given the infusion by mistake. A decision to exclude was made the same day.
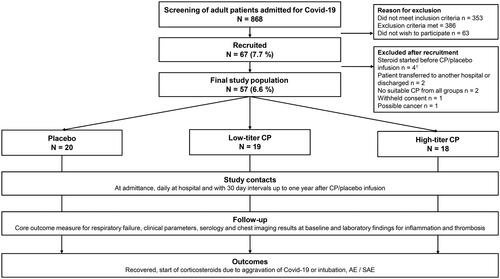
Table 1. Baseline characteristics.
Table 2. Comparison of primary and secondary outcomes between randomised and ad hoc groups.
Table 3. Adverse (AE) and severe adverse events (SAE) for hospitalised COVID-19 patients receiving convalescent plasma containing a high (HCP) or low (LCP) titre of anti-SARS-CoV-2 neutralising antibodies, or placebo.
