Figures & data
Figure 1. Comprehensive management of COPD.
Integrated approach to care that includes confirming COPD diagnosis with spirometry, evaluation of symptom burden and risk of exacerbations with on-going monitoring, assessment for features of asthma, and comprehensive management, both non-pharmacologic and pharmacologic.
* = Self-Management Education includes appropriate inhaler device technique and review, breathing techniques and review, early recognition of AECOPD, written action plan development and implementation (if appropriate).
mMRC is a modified (0-4 scale) version of the MRC breathlessness scale which was used in previous CTS guidelines. The mMRC aligns with the Global Initiative for Chronic Obstructive Airways Disease (GOLD) 2019 report.
Abbreviations: CAT = COPD assessment test; mMRC = Modified Medical Research Council; prn = as-needed; AECOPD = acute exacerbation of COPD; Inhaled Long-Acting Therapies = long-acting muscarinic antagonist and/or long-acting ẞ2-agonist and/or inhaled corticosteroid; NIV = non-invasive ventilation.
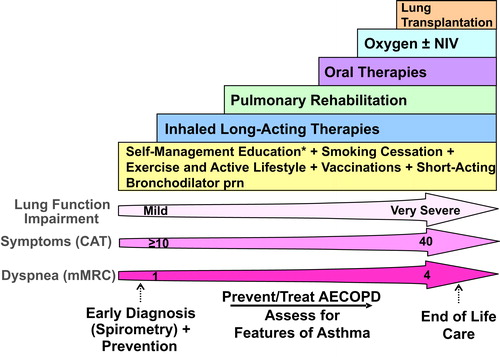
Figure 2. COPD Pharmacotherapy.
COPD pharmacotherapy promoting an approach that aligns treatment decisions with symptom burden and risk of future exacerbations. To learn more about the Asthma-COPD Overlap (ACO) treatment algorithm, refer to the CTS position statement on the pharmacotherapy in patients with COPD in 2017.Citation1
mMRC is a modified (0-4 scale) version of the MRC breathlessness scale which was used in previous CTS guidelines. The mMRC aligns with the Global Initiative for Chronic Obstructive Airways Disease (GOLD) 2019 report.
SABD prn (as needed) should accompany all recommended therapies. Solid arrows indicate step up therapy to optimally manage symptoms of dyspnea and/or activity limitation, as well as prevention of AECOPD where appropriate. Dashed arrows indicate potential step down of therapy, with caution and with close monitoring of patient symptoms, exacerbations and lung function. Symbol “/” refers to combination products (in the same device) and combination regimens (in separate devices). ICS should ideally be administered in a combination inhaler.
†Patients are considered at Low Risk of AECOPD with ≤1 moderate AECOPD in the last year (moderate AECOPD is an event with prescribed antibiotic and/or oral corticosteroids), and did not require hospital admission/ED visit; or at High Risk of AECOPD with ≥2 moderate AECOPD or ≥1 severe exacerbation in the last year (severe AECOPD is an event requiring hospitalization or ED visit).
*Blood eosinophil ≥300/µL in patients with previous AECOPD may be useful to predict a favorable response to ICS combination inhaler.
‡Oral Therapies = Roflumilast, N-acetylcysteine, daily dose Azithromycin could be considered with patients with high risk AECOPD despite on optimal long-acting inhaled therapy. Oral corticosteroids as maintenance therapy are not indicated in COPD.
Abbreviations: CAT = COPD assessment test; mMRC = Modified Medical Research Council; SABD prn = short-acting bronchodilator as needed; AECOPD = acute exacerbation of COPD; LAMA = long-acting muscarinic antagonist; LABA = long-acting ẞ2-agonist; ICS = inhaled corticosteroid.
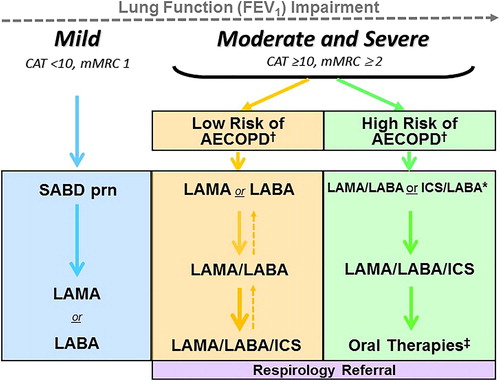
Table 1. 2019 Recommendations on improving symptoms, exercise tolerance, physical activity and health status in stable COPD patients.
Table 2. 2019 Recommendations on preventing acute exacerbation in stable COPD.
