Figures & data
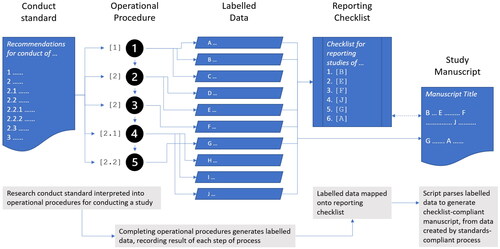
Figure 1. Operationalisation into the general protocol of COSTER Recommendation 1.1.2: ‘identify information management practices for each stage of the review, including reference and knowledge management tools, systematic review software, and statistics pakages’.
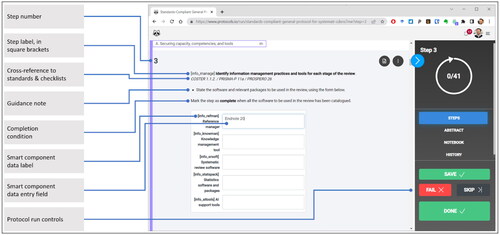
Table 1. Cross-walk of the top-level data labels and steps of the general-purpose protocol with items in the conduct standards and reporting checklists with which the protocol is designed to facilitate compliance.
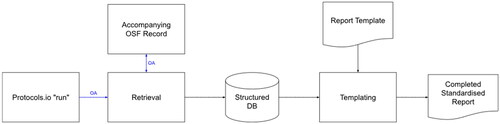
Figure 2. The process for automating the documentation of a protocol run. Arrows in blue represent interfaces secured using the Oauth2 authentication standard (https://oauth.net/2/).
Data availability statement
All data is available from the accompanying OSF project record at https://doi.org/10.17605/OSF.IO/ZJEVP and GitHub repository at https://github.com/StephenWattam/TARPD