Figures & data
Figure 1. (A) A schematic showing the transplantation site of a bioartificial graft. The cutout shows the anticipated composition of a bioartificial organ graft for the treatment of diabetes, revealing microencapsulated islets, sertoli cells and hepatocytes in a single immunoisolation device. (B) A photomicrograph of a bioartificial islet graft composed of reversibly immortalized human beta, alpha and sertoli cells in a microcapsule, transplanted intraportally.
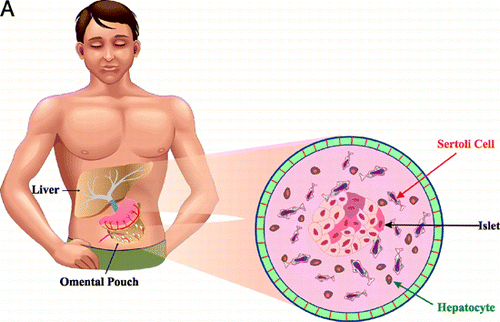
Table 1. Summary of Public and Private Bioartificial Pancreas Research (1980–2001)
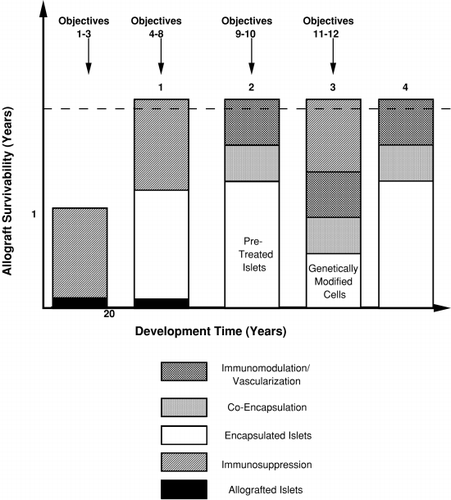
Figure 2. The evolution of a clinical therapy for diabetes based on a bioartificial pancreas. The earliest strategy will involve the use of encapsulated allografted islets, under immunosuppression, with graft prolongation due to co‐encapsulation as well as genetic modifications to the islets which provide immunomodulation and vascularization Strategy 1 is basically an immunoprotected extension of the Edmonton Protocol (3) which rendered brittle diabetics insulin independent for approximately twelve months. It is based on over two decades of development. Eventually, due to optimization of islet handling and pretreatment, co‐encapsulation and in situ graft protection, the need for immunosuppression could be eliminated (Strategy 2). Given the ultimate requirement of replacing allotransplantation with cultured tissue to treat all type I diabetics, genetically modified cell lines will be developed and applied in combination with the approaches followed in Strategies 1 and 2. Within three decades genetically modified cells should replace islets, first with immunosuppression (Strategy 3) and ultimately without (Strategy 4).