Figures & data
Figure 1. Interaction of liposomes and platelets detected by flow cytometry. A) CF‐liposomes interacted with washed platelets in buffer. Green fluorescence intensity is shown on the log scale of the x–axis; cell numbers on the y‐axis. The open histogram is the control platelet population; the dark histogram shows the level of fluorescence of the platelet population incubated with CF‐liposomes; the degree of fluorescence conferred by an equal amount of free CF is the gray histogram. B) Fluorescence of mouse platelets in plasma in response to FITC‐PE labeled liposomes is shown. The open histogram is the control platelet population; the dark histogram is the fluorescence of mouse PRP interacted with lipid‐labeled liposomes.
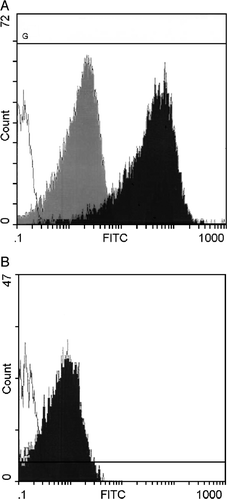
Figure 2. The fluorescence pattern of 106/mL platelets labeled with anti‐CD42b‐R in the presence of CF‐liposomes (lipid 10 nmol/mL, CF 17 pmol/mL) in buffer. The x–axis quantitates red phycoerythrin (PE) florescence and the y‐axis, green CF fluorescence, on a log scale in arbitrary units.
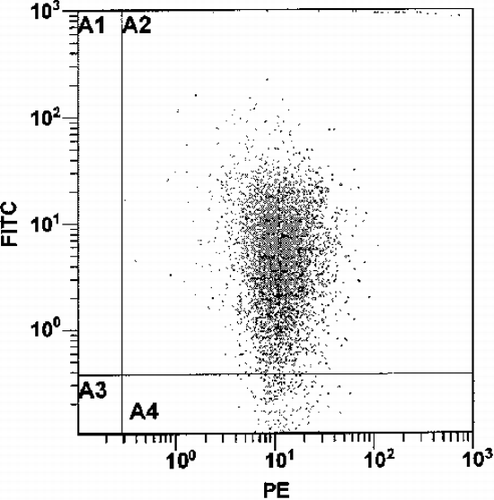
Table 1. Analysis of Fluorescence Associated with the Platelet and Liposome Fractions Separated by Gel Exclusion Chromatography
Figure 3. The dose‐response curve of platelets (106/mL) in buffer with liposomes labeled with: CF (solid line) (molar ratio of lipid:CF, 760:1); R18 (broken line) (molar ratio of lipid:R18, 135:1). The x‐axis represents the amount of liposomes (lipid nmol/mL); the y‐axis represents the percentage of fluorescent (red or green) labeled platelets.
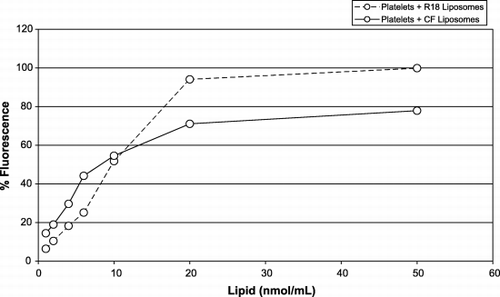
Table 2. Liposomes of a Range of Compositions Interact with Platelets in Plasma or Whole Blood
Figure 4. Interaction of platelets and liposomes in plasma (triangles) or buffer (squares) and in the presence of anti‐CD42b‐R on platelets’ surface in plasma (solid symbols) or in buffer (open symbols). The x‐axis represents the amount of CF‐liposomes, lipid nmol/mL (molar ratio of lipid:CF, 760:1); the y‐axis represents the percentage of fluorescent platelets (106/mL).
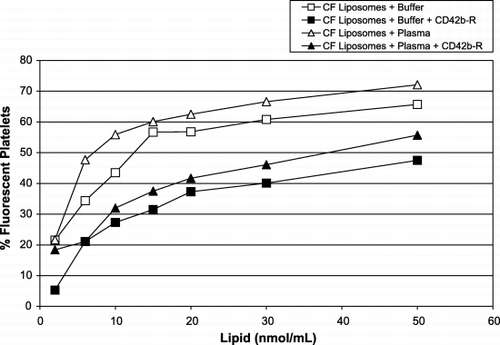
Figure 5. Fluorescence of cells in WB induced by the presence of increasing concentrations of CF‐liposomes (molar ratio lipid/CF, 760:1): leukocytes (triangles); red cells (squares); and platelets (circles). The x‐axis represents the amount of CF‐liposomes, lipid nmol/mL (molar ratio of lipid:CF, 760:1); the y‐axis represents the percentage of fluorescent blood cells.
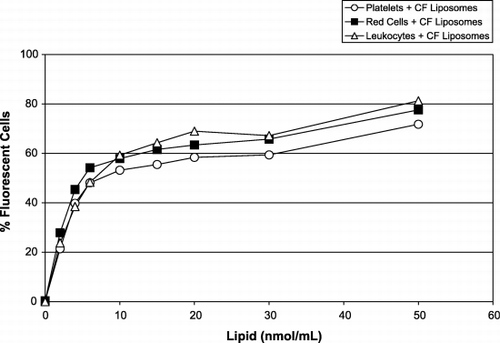
Figure 6. Leukocyte fluorescence in WB. Increasing concentrations of liposome formulations 1 (circles) and 3 (squares) were added and the leukocytes were identified by anti‐CD45‐PC5. The x‐axis represents L of 10 mM liposome lipid added to a platelet population; the y‐axis shows the percentage of the leukocyte population positive for FITC‐PE.
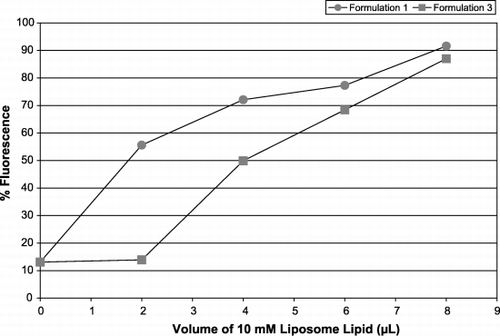
Figure 7. Fluorescence of leukocyte‐platelet aggregates in WB. Increasing concentrations of liposome formulations 1 (circles) and 3 (squares) were added and the leukocyte‐platelet aggregates were selected by anti‐CD45‐PC5, interrogated for CD42b‐R then assessed for the presence of FITC‐PE. The x‐axis represents L of 10 mM liposome lipid added to a platelet population; the y‐axis shows the percentage of the leukocyte population positive for FITC‐PE.
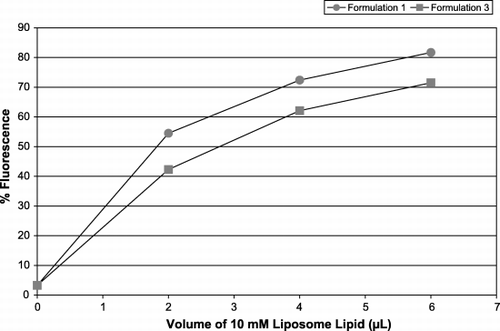
Figure 8. Time course of liposome‐blood cell interaction in WB. Liposomes labeled with CF (solid lines) or R18 (broken lines) were added to whole blood and samples were removed at the indicated intervals. Red cells (closed symbols) and platelets (open symbols) were assessed for fluorescence. Liposomes with PEG2000 (squares) or without (circles) were compared. The x‐axis represents time in seconds; the y‐axis shows the % fluorescence of the cell population.
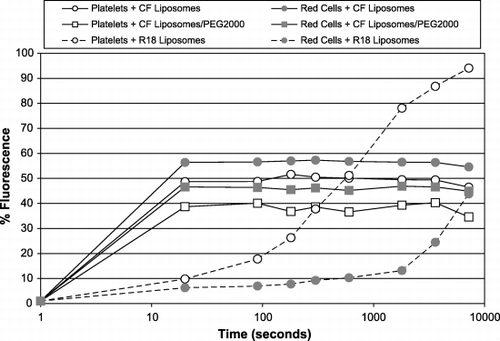
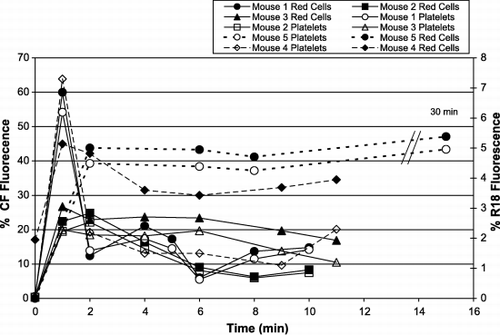
Figure 9. Intravenous injection of fluorescent liposomes into mice. Different lots of liposomes containing CF (solid lines) or R18 (broken lines) were injected into mice and the fluorescence of platelets and red cells was monitored. The x‐axis shows the times of blood samples taken after the injection; the y‐axes are % florescence of the cell populations: left y‐axis ‐ CF fluorescence; right ‐ R18. The platelet populations are shown by open symbols, and the red cells by closed symbols.