Figures & data
Figure 1. Viability by Trypan blue staining (n = 10). Cell viability decreased over time, from 93.7 ± 2.21% at PT0 to 90.2 ± 2.35% at PT10, 87.5 ± 3.06% at PT20, and 84.8 ± 2.94% at PT30 (P < 0.01 for all time points).
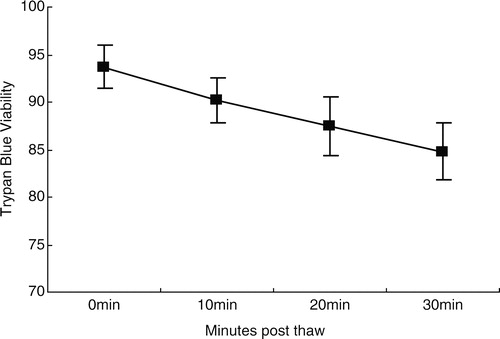
Figure 2. Flow cytometric analysis of apoptotic marker expression in post-thaw UCB samples. The gating of CD34+ cells (P2) (A) and CD45+ cells (P1) (B) from UCB samples after thawing. Gated CD34+ cells (P2) at time PT0 (C), 10 (D), 20 (E), and 30 minutes (F) were analyzed for apoptotic markers as assessed by staining with Ann V and PI. Gated CD45+ cells (P1) from UCB samples at time PT0 (G), 10 (H), 20 (I), and 30 minutes (J) were also analyzed for apoptotic markers.
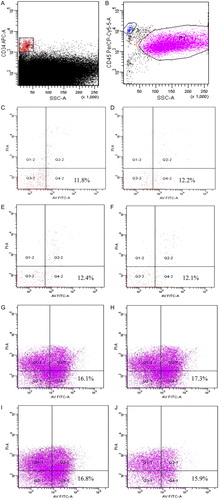
Figure 3. The percentage of Ann V(+) binding to CD34+ PI(−) cells and CD45+ PI(−) cells in UCB samples (n = 10). The number of early apoptotic CD34+ PI(−) cells and CD45+PI(−) cells was similar over time, and the differences were not significant (P > 0.05 for both cell markers). (▴) Ann V(+)PI(−) CD34+ cells; (▪) Ann V(+)PI(−)CD45+ cells.
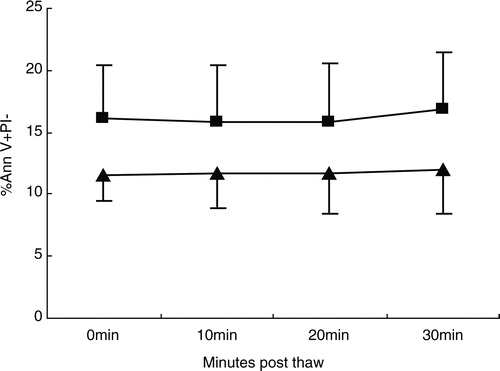
Figure 4. (A) The ability to form CFUs (relative to prefreeze content) was 85.2 ± 13.09% after thawing at PT0, which demonstrated a significant decrease (P = 0.01). (B) The number of CFUs was 104 ± 18.8, 101 ± 17.5, 101 ± 18.8, and 98 ± 19.3, respectively, at PT0, 10, 20, and PT30, at time point PT30, the number of CFUs was decreased in comparison to PT0 (P < 0.05), but the number of CFUs was unchanged within 20 minutes (P > 0.05).
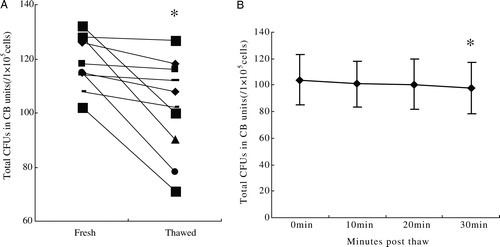
Table 1. Characteristics of patients
Table 2. Toxicity associated with 10 UCB infusions
