Figures & data
Figure 1. Systematic research methodology to identify company sponsored clinical trials relating to each new medicine and to assess the disclosure of results. IFPMA = International Federation of Pharmaceutical Manufacturers and Associations; EPAR = European Public Assessment Report.
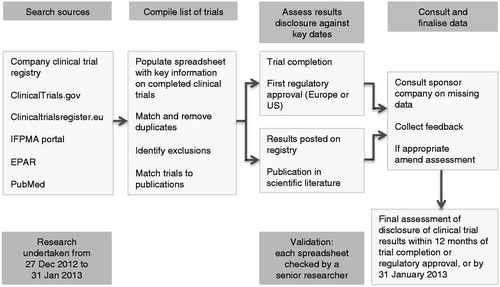
Table 1. Total number of clinical trials posted on each registry relating to the 53 new medicines approved by the EMA in 2009, 2010 and 2011.
Table 2. Number of completed company-sponsored clinical trials relating to 53 new medicines approved in 2009, 2010 and 2011 which had disclosed results, grouped by phase of study.
Table 3. Number of completed company-sponsored clinical trials relating to 21 new medicines approved in 2009 which had disclosed results, grouped by phase of study.
Table 4. Number of completed company-sponsored clinical trials relating to 12 new medicines approved in 2010 which had disclosed results, grouped by phase of study.
Table 5. Number of completed company-sponsored clinical trials relating to 20 new medicines approved in 2011 which had disclosed results, grouped by phase of study.