Figures & data
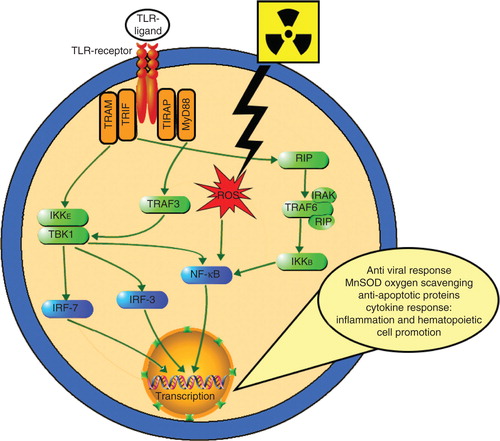
Figure 1. Radiation countermeasures under development. Currently, there are seven radiation countermeasures that have US FDA IND status: Androstenediol (5-AED), BIO 300, CBLB502, Ex-RAD, HemaMax, Neupogen and OrbeShield. Neupogen and Leukine are expected to obtain US FDA Emergency Use Authorization and both are available in the SNS. Promising molecules at different stages of development are presented under different groups.
Table 1. Promising radiation countermeasures in advanced stages of development with US FDA IND status*.
Table 2. Radiation countermeasures already in SNS that may receive US FDA EUA status.
Table 3. Other promising radiation countermeasures at advanced stages of development requiring US FDA IND status.
Table 4. Promising radiation countermeasures in the early stages of development.
Table 5. Patents for radiation countermeasures (protectors, mitigators and therapeutics/treatments).
Figure 2. Brief diagrammatic representation of radiation injury and the mode of action of radiation countermeasures at advanced stages of development. This simplified response pathway of a subject’s irradiation shows that radiation exposure induces free radicals, DNA breaks and apoptosis. The various radiation countermeasures reduce the injurious effects of irradiation through different pathways as indicated by colored arrows. Only the drugs with well-understood mechanism of action are included and may have been indicated at multiple points, as several drugs work through several pathways. Red arrows indicate inhibition of deleterious effects of radiation injury and green arrows indicate enhancement of recovery.
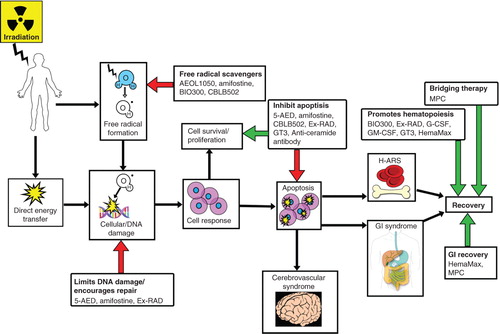
Figure 3. Schematic representation of TLR-ligand-mediated NF-κB activation. TLR ligands (multiple, not all radioprotective) interact with TLR receptor inducing two divergent signaling pathways controlled by two pairs of adaptor proteins: TRAM/TRIF and TIRAP/MyD88. The MyD88-dependent pathway quickly upregulates inflammatory cytokines via NF-κB activation by its dissociation from inhibitory component (IκB). This permits NF-κB to enter the nucleus where it can ‘turn on’ the expression of specific genes such as inflammatory or immune response, a cell survival response, cellular proliferation and oxygen-scavenging MnSOD. The MyD88-independent pathway does this as well, in addition to inducing type-1 IFN expression through IRFs and triggering IFN-β, which results in cell maturation. MyD88-independent pathways are activated with slower kinetics. Radiation produces ROS, which also activate NF-γB.