Figures & data
Figure 1. HCV genome structure and vaccine immunogens. (A) Organisation of the HCV genome: HCV, a single-stranded RNA virus of ∼ 9.5 kb, consists of a single open-reading frame and two untranslated regions. HCV is transcribed as a single polyprotein, which is cleaved by a host signal protease in the structural region and the HCV-encoded serine protease in the NS region. The hypervariable regions of E2 (HVR1 + HVR2) are indicated by dashed arrows. The protein products of cleavage are shown. The structural regions consist of core and the two envelope proteins, gp35 and gp76. The NS proteins are shown and their functions are described where known. (B) Prophylactic vaccines for HCV tested in primates (including man) are listed according to the lead author of the paper in which they are described. The relative coverage of the HCV genome by vaccine immunogen is shown. The genotype of the immunogen encoded in each vaccine is shown in parenthesis with the paper reference [].
![Figure 1. HCV genome structure and vaccine immunogens. (A) Organisation of the HCV genome: HCV, a single-stranded RNA virus of ∼ 9.5 kb, consists of a single open-reading frame and two untranslated regions. HCV is transcribed as a single polyprotein, which is cleaved by a host signal protease in the structural region and the HCV-encoded serine protease in the NS region. The hypervariable regions of E2 (HVR1 + HVR2) are indicated by dashed arrows. The protein products of cleavage are shown. The structural regions consist of core and the two envelope proteins, gp35 and gp76. The NS proteins are shown and their functions are described where known. (B) Prophylactic vaccines for HCV tested in primates (including man) are listed according to the lead author of the paper in which they are described. The relative coverage of the HCV genome by vaccine immunogen is shown. The genotype of the immunogen encoded in each vaccine is shown in parenthesis with the paper reference [].](/cms/asset/8119cc16-6003-420a-85d6-251ef7d7757a/iebt_a_791277_f0001_b.jpg)
Table 1. Primate and human studies describing candidate prophylactic HCV vaccines.
Figure 2. Magnitude of T-cell response to vaccination. A comparison of the magnitude of vaccine-induced T-cell responses in primates is shown (median of peak response after vaccination as measured by IFNγ ELISpot). Vaccines contained HCV antigens unless otherwise stated. Successive vaccinations are separated by a forward slash, e.g., DNA/MVA refers to a vaccine regimen using DNA priming followed by an MVA boost. References in parenthesis [].
![Figure 2. Magnitude of T-cell response to vaccination. A comparison of the magnitude of vaccine-induced T-cell responses in primates is shown (median of peak response after vaccination as measured by IFNγ ELISpot). Vaccines contained HCV antigens unless otherwise stated. Successive vaccinations are separated by a forward slash, e.g., DNA/MVA refers to a vaccine regimen using DNA priming followed by an MVA boost. References in parenthesis [].](/cms/asset/d5d4c027-7b85-42f7-b93f-62c826257005/iebt_a_791277_f0002_b.jpg)
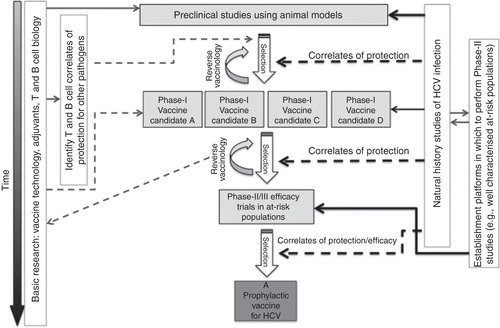
Figure 3. Progress to an effective prophylactic vaccine against HCV. A summary of some of the key interactions between basic research and vaccine studies is shown. Natural history studies of HCV infection are used to better understand the immune correlates of protection against HCV infection; this understanding informs all levels of vaccine design, in particular the design of vaccines for preclinical and Phase I studies and at the selection stages when assessing efficacy of candidate vaccines. Cohorts of at-risk populations need to be characterised before candidate vaccines can be tested in Phase II/III studies and so this work should be done in parallel with early-stage vaccine assessment. Phase II/III studies of vaccine efficacy may be required to further define correlates of protection for optimal vaccine generation – a process termed “reverse vaccinology”. Basic research into vaccine modalities, adjuvants and the biology of T and B cells can be fed into the process of vaccine development at all stages to allow us to better design, assess and implement novel vaccines.