Figures & data
Figure 1. (A) US FDA-approved products (from 1996 to 2010), (B) Top global biopharmaceutical producers in 2011 (based on revenue), and (C) Global revenue forecast for biosimilars.
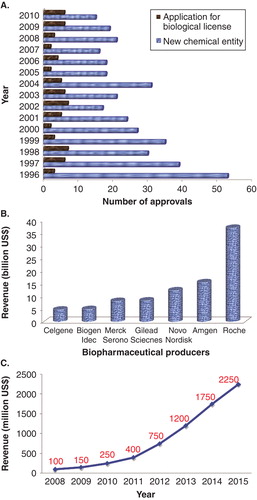
Figure 2. (A) Steps involved in the manufacturing process of biosimilars, and (B) Challenges involved in developing biosimilars.
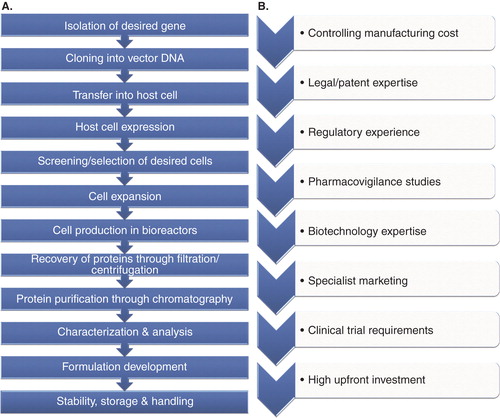
Table 1. Differences between small-molecule drugs/generics and biologics/biosimilars Citation[9].
