Figures & data
Table 1. Stem-cell–based cardiac resynchronization studies.
Figure 1. Stem-cell intervention rescues disparity in ventricular wall motion post-infarction. Impact of stem-cell biotherapy on cardiac dyssynchrony deconvoluted in a murine infarction model. A total dose of 200,000 undifferentiated induced pluripotent stem (iPS) cells per heart (40,000 cells/site × 5 sites) was delivered by epicardial route into the peri-infarcted anterior wall of the left ventricle within 30 min following coronary ligation. Pre-infarction, all segments of the left ventricle demonstrate harmonious contraction during systole (left top) and relaxation during diastole (left middle) documented by in vivo speckle-tracking echocardiography. At 1 month, infarction precipitated dyssynchronous motion characterized by early stretch followed by delayed contraction (middle) with correction afforded by iPS cell therapy (right). Bottom row depicts fitted strain patterns reflecting normokinesis pre-infarction (left), dyssynchrony post-infarction without treatment (middle), and resynchronization following cell therapy (right). See also Ref. Citation[10].
![Figure 1. Stem-cell intervention rescues disparity in ventricular wall motion post-infarction. Impact of stem-cell biotherapy on cardiac dyssynchrony deconvoluted in a murine infarction model. A total dose of 200,000 undifferentiated induced pluripotent stem (iPS) cells per heart (40,000 cells/site × 5 sites) was delivered by epicardial route into the peri-infarcted anterior wall of the left ventricle within 30 min following coronary ligation. Pre-infarction, all segments of the left ventricle demonstrate harmonious contraction during systole (left top) and relaxation during diastole (left middle) documented by in vivo speckle-tracking echocardiography. At 1 month, infarction precipitated dyssynchronous motion characterized by early stretch followed by delayed contraction (middle) with correction afforded by iPS cell therapy (right). Bottom row depicts fitted strain patterns reflecting normokinesis pre-infarction (left), dyssynchrony post-infarction without treatment (middle), and resynchronization following cell therapy (right). See also Ref. Citation[10].](/cms/asset/34a90b54-d441-498a-af0c-fcb13a5bb007/iebt_a_922536_f0001_b.jpg)
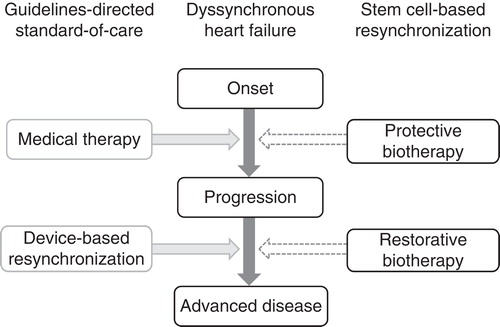
Figure 2. Stem-cell–based resynchronization complements standard of care. Dyssynchronous heart failure is a malignant disorder commonly refractory to the existing therapeutic armamentarium that currently combines pharmacotherapy with device-based resynchronization. Responsiveness to pacing devices is impeded by the scar burden post-infarction, mandating approaches capable to promote tissue repair. Potential applications of stem-cell–based reparative resynchronization include cardioprotection in acute/subacute phases of disease to prevent disease progression, and normative restitution to restore structure and function in the setting of chronic dyssynchronous heart failure.