Figures & data
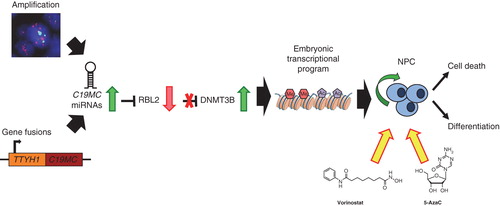
Figure 1. Mechanisms of DNMT-mediated gene silencing. Activating methylation of H3K4 and acetylation of histone tails promotes a euchromatic state, enabling transcription factor binding, and activates transcription of corresponding gene. DNMT and HMT-mediated methylation of H3K9 and H3K27 results in recruitment of chromatin remodeler MBD proteins, which subsequently recruits HDAC complexes for the removal of acetyl groups from histone tails. This results in chromatin condensation, which prevents transcription factor binding and promotes gene silencing.
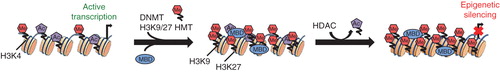
Figure 2. Targeting DNMTs as novel therapeutics in high-risk CNS-PNETs. C19MC amplification or TTYH1-C19MC gene fusions result in overexpression of C19MC miRNAs. C19MC-mediated post-translational downregulation of RBL2 leads to de-repression of DNMT3B and high expression of an embryonic, brain-specific DNMT3B isoform that promotes a highly primitive neural transcriptional and epigenomic program in CNS-PNETs. Treatment with DNMT or HDAC inhibitors, 5-AzaC and Vorinostat, inhibits growth of cell lines from C19MC-associated lethal embryonal brain tumors by promoting cell death or differentiation.