Figures & data
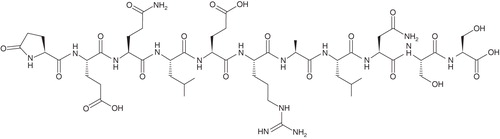
Box 1. Drug summary.
Figure 2. Pharmacokinetic data obtained in healthy volunteers. A. Male subjects receiving subcutaneous ARA 290 at doses 2 mg (n = 10), 4 mg (n = 5) and 6 mg (n = 5). B. Male subjects receiving an intravenous dose of 2 mg (n = 10). Values are mean ± SEM.
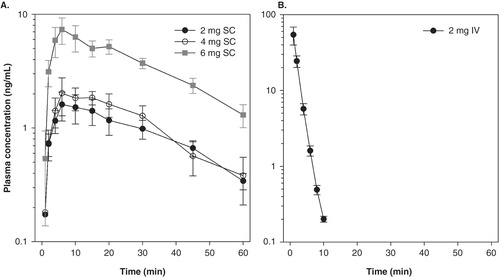
Figure 3. Effect of treatment with ARA 290 treatment for 15 weeks (weeks 1 and 2: 0.03 mg/kg at a 2-day interval, weeks 3–15: 0.03 mg/kg once weekly) on allodynia in a spared nerve injury rat model. Allodynia was measured using von Frey filaments; the force (in grams) causing a withdrawal response of the affected paw is given on the y-axis. Open squares are sham-operated animals, open triangles vehicle-treated animals, closed circles ARA 290-treated animals (n = 8/group). The closed triangles denote the ARA 290 treatment days. Values are mean ± SEM.
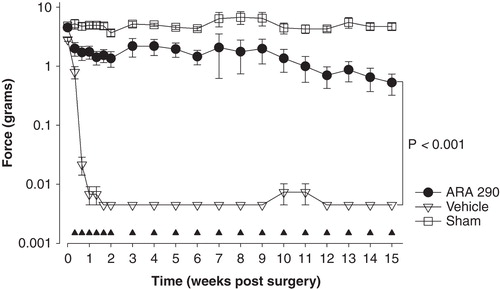
Table 1. Study design of trial #1, An open-label study on the effect of a 1-week treatment with intravenous ARA 290 on pain scores in chronic neuropathic pain patients.
Table 2. Patient characteristics of trial #1 (see ).
Figure 4. Effect of ARA 290 treatment on neuropathic pain in 10 sarcoidosis patients (A) and 10 patients with diabetes mellitus (B). Responders are defined as patients with a reduction in pain score ≥ 2 points within the initial 10 days following the start of treatment. Star: p < 0.05 vs. baseline pain score (analysis on all patients). The closed triangles denote the ARA 290 treatment days. Values are mean ± SEM. The broken grey lines are the 30% and 50% response lines. For study design see and for patient characteristics see .
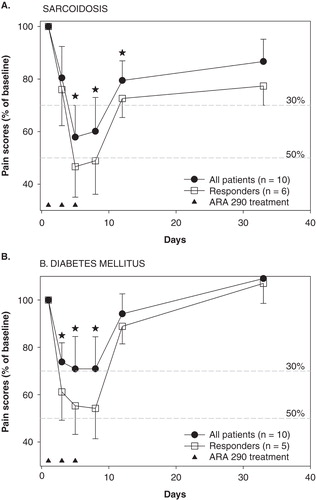
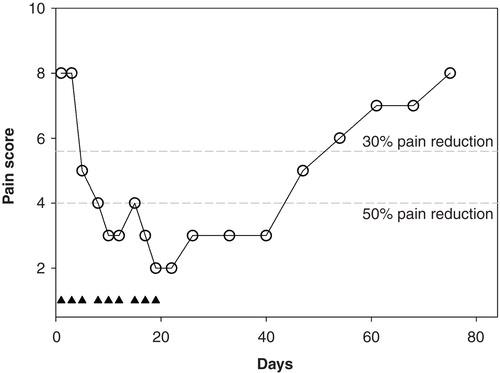
Figure 5. Effect of a 3-week ARA 290 treatment in one sarcoidosis patient with severe neuropathic pain. The closed triangles denote the ARA 290 treatment days. The broken grey lines are the 30% and 50% response lines.