Figures & data
Table 1. Genes associated with FALS.
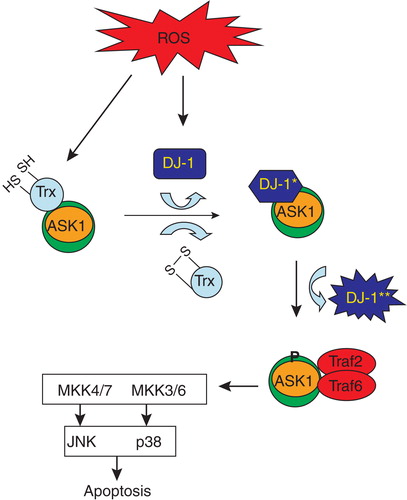
Figure 1. Hypothetical scheme for activation of ASK1 by oxidative stress. In resting conditions, ASK1 is bound to and negatively regulated by TRX. Under conditions of oxidative stress, TRX becomes oxidized and dissociates from the ASK1 signaling complex (signalosome). DJ-1 becomes oxidized under conditions of oxidative stress (*) and subsequently binds to ASK1, negatively regulating its activity. Continued oxidation of DJ-1 can lead to a conformational change (**) where it dissociates from the ASK1 signalosome, relieving the negative inhibition. ASK1 becomes phosphorylated and is now activated resulting in the activation of JNK and p38 pathways. This schematic connects ASK1, DJ-1 and p38 targets in a common pathway initiated by oxidative stress.