Figures & data
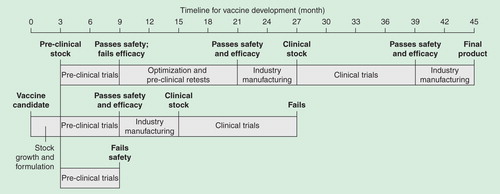
Figure 1. Hypothesized transmission of MERS-CoV from animal hosts to humans. (A) MERS-CoV is potentially transmitted by infected bats to African one-humped camels, which are often exported to the Arabian Peninsula. (B) Vaccination of one-humped camels could, therefore, prevent further transmission of the virus to humans and subsequent human-to-human transmission if one-humped camels are indeed the primary route of infection for humans.
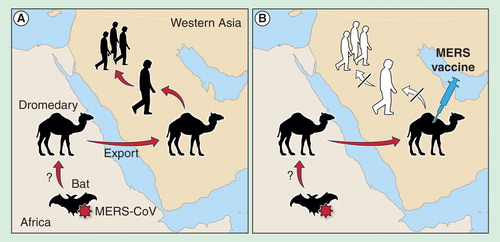
Figure 2. Estimation of major hospital costs affiliated with a MERS outbreak. Average cost per day per in-patient Citation[147,148] was multiplied by the median number of days for total cost per treatment. In-patient stay: US average of US$3145/day × 14 days median for a MERS in-patient = US$44,030.00; intensive care unit stay: †US$16,474 × 22 days = US$362,430; mechanical ventilation: †US$23,750 × 11.5 days = US$273,139; renal replacement therapy: US$3819 × 7 days = US$26,734; total: sum of in-patient costs after multiplying by the percentage required and adding the additional administrative costs of US$79,150 per in-patient = US$713,942. An in-patient requiring all interventions would incur expenses of more than US$785,000.
![Figure 2. Estimation of major hospital costs affiliated with a MERS outbreak. Average cost per day per in-patient Citation[147,148] was multiplied by the median number of days for total cost per treatment. In-patient stay: US average of US$3145/day × 14 days median for a MERS in-patient = US$44,030.00; intensive care unit stay: †US$16,474 × 22 days = US$362,430; mechanical ventilation: †US$23,750 × 11.5 days = US$273,139; renal replacement therapy: US$3819 × 7 days = US$26,734; total: sum of in-patient costs after multiplying by the percentage required and adding the additional administrative costs of US$79,150 per in-patient = US$713,942. An in-patient requiring all interventions would incur expenses of more than US$785,000.](/cms/asset/dc123d44-442f-407f-add5-2f281303861b/ierv_a_1036033_f0002_ob.jpg)
Table 1. Functions of nonstructural, major structural and accessory structural proteins of Middle East respiratory syndrome coronavirus.
Figure 3. Idealized vaccine development timeline from post-discovery to pre-regulatory submission. A simplified timeline illustrates the potential pitfalls encountered throughout the development process. Optimistic estimates for vaccine development from candidate selection to industrial production fall between 3.5 and 4 years, depending on the type of vaccine. After adding 2–3 years for research prior to candidate selection and 2–3 years for regulatory submission and licensure once a final formulation is in hand, total time is approximately 10 years. As discovery methods and bureaucratic processes and approvals are accelerating, the overall timeline could realistically shrink to 6–7 years.