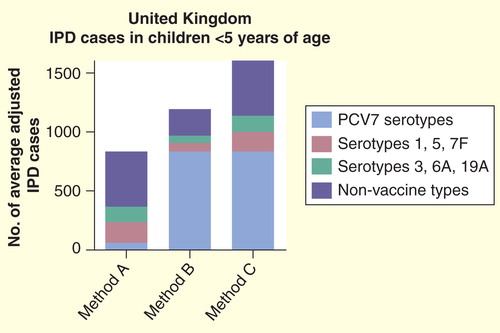
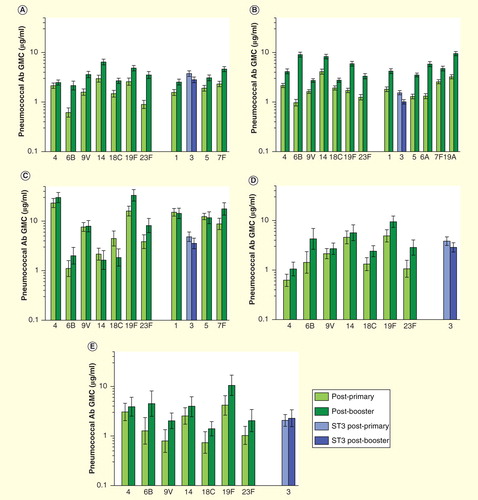
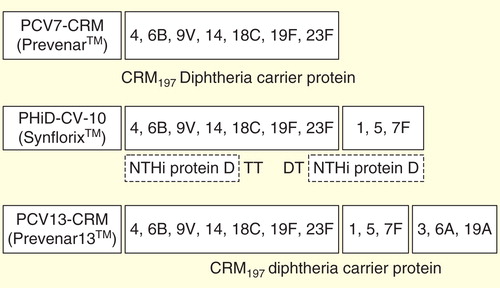
Figures & data
Table 1.
Efficacy of vaccination with four pneumococcal conjugate vaccine or vaccine candidates in infants and young children against vaccine type and serogroup 6 acute otitis media as assessed in tympanocentesis-based double-blind randomized controlled studies.
Table 2.
Lack of obvious relationship between serotype-specific immunogenicity and efficacy against vaccine type acute otitis media.
Table 3.
Trend in 19A cases: comparison pre- versus post-PHiD-CV-10 implementation in vaccine-eligible children (countries reporting at least 5 cases of 19A annually pre-PHiD-CV-10).