Figures & data
(A) Different Escherichia coli strains (BL21 with and without a co-expression plasmid harboring the chaperone GroEL/ES [GroE7]) were analyzed for soluble expression by SDS-PAGE (left lane for each construct is soluble homogenate and the right is the eluate from immobilized metal-affinity chromatography purification). Two different constructs (denoted G25 and G26, with different domain boundaries of human 11β-HSD1) were used to inoculate the cultures. After initial incubation, cultures were split and treated with CBX or solvent (DMSO). Arrow indicates the expected mass of 11β-HSD1. (B) Ligand supplementation of human 11β-HSD1 with distinct chemical scaffolds (cpd 1, cpd 2, 18α-GA and 18β-GA). Different 11β-HSD1 inhibitors were used in analogous expression experiments, as presented for CBX (A). Note the specificity for the GA isomers 18α-GA and 18β-GA.
CBX: Carbenoxolone; ctrl: Control; GA: Glycerrhitinic acid.
![Figure 1. Ligand supplementation (100 µM CBX during induction phase) leads to increased soluble expression of distinct human short-chain dehydrogenases/reductases, shown here by expression of type 11β-hydroxysteroid dehydrogenase (HSD).(A) Different Escherichia coli strains (BL21 with and without a co-expression plasmid harboring the chaperone GroEL/ES [GroE7]) were analyzed for soluble expression by SDS-PAGE (left lane for each construct is soluble homogenate and the right is the eluate from immobilized metal-affinity chromatography purification). Two different constructs (denoted G25 and G26, with different domain boundaries of human 11β-HSD1) were used to inoculate the cultures. After initial incubation, cultures were split and treated with CBX or solvent (DMSO). Arrow indicates the expected mass of 11β-HSD1. (B) Ligand supplementation of human 11β-HSD1 with distinct chemical scaffolds (cpd 1, cpd 2, 18α-GA and 18β-GA). Different 11β-HSD1 inhibitors were used in analogous expression experiments, as presented for CBX (A). Note the specificity for the GA isomers 18α-GA and 18β-GA.CBX: Carbenoxolone; ctrl: Control; GA: Glycerrhitinic acid.](/cms/asset/273e0079-da2a-4005-aba2-3d84e05584c3/ieru_a_11217000_f0001_b.jpg)
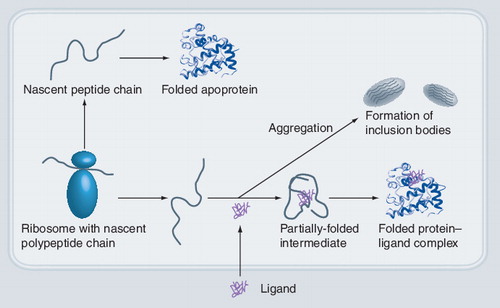
Nascent polypeptides can fold into apoproteins or aggregate and are then deposited in inclusion bodies. Inclusion of a ligand can promote and stabilize the folded protein, leading to a stable and soluble protein–ligand complex.