Figures & data
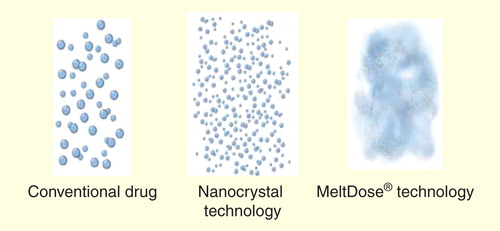
Figure 2. Depiction of the absorption of MeltDose® formulation of tacrolimus throughout the gastrointestinal tract across 24 h.
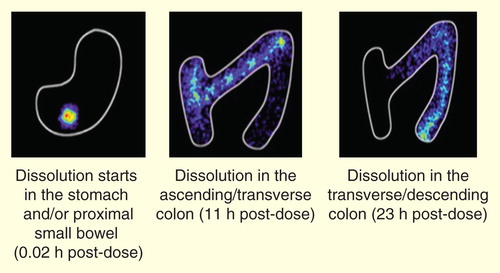
Figure 3. Pharmacokinetic profiles of twice-daily and once-daily tacrolimus formulations. (A) Mean whole-blood tacrolimus concentration in stable kidney transplant patients on tacrolimus twice-daily (Day 7) converted to LCP-Tacro (Day 14) versus time. (B) Mean whole blood tacrolimus concentrations in stable liver transplant patients on tacrolimus twice daily (Day 7) converted to LCP-Tacro (days 14, 21 and in week 26) versus time.
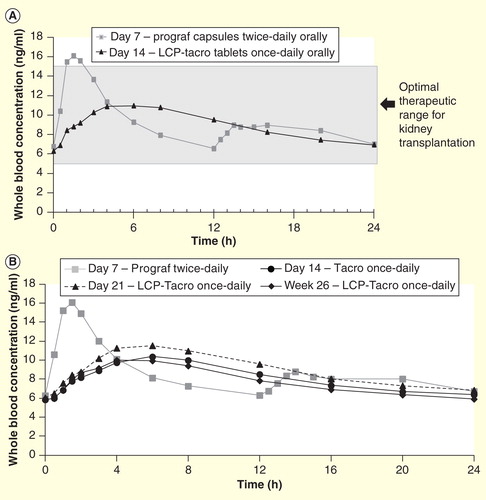
Table 1. Summary of PK results from Phase II trials.
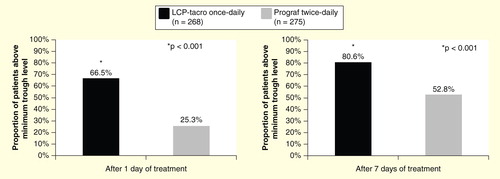
Table 2. Summary of Phase II trial efficacy results.
Figure 5. Cumulative dose adjustments. (A) During the initial 14 days of dosing (B) over the 12 months study period in de novo kidney transplant recipients assigned to LCP-Tacro or tacrolimus twice-daily.
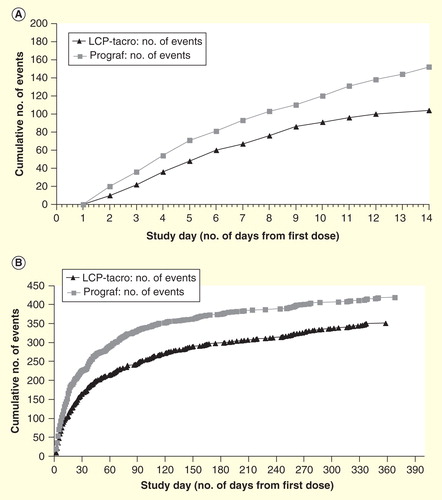
Table 3. Summary table of Phase III trial results.
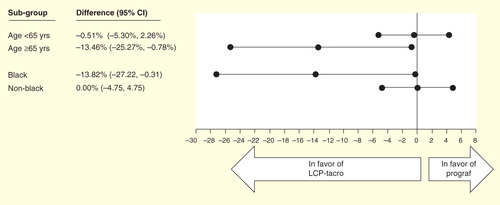
Figure 6. Efficacy at 1-year post-transplant in de novo kidney transplant recipients randomized to LCP-Tacro or tacrolimus twice-daily Citation[46].
![Figure 6. Efficacy at 1-year post-transplant in de novo kidney transplant recipients randomized to LCP-Tacro or tacrolimus twice-daily Citation[46].](/cms/asset/f6c90e81-aacb-41db-8fbc-159d0aa616b4/ierm_a_983903_f0006_b.jpg)
Table 4. Adverse events reported in Phase III trials of LCP-Tacro.
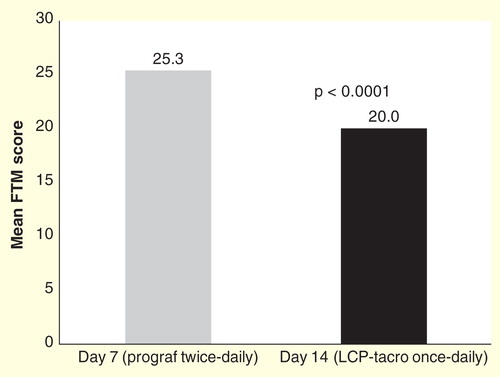
Figure 8. Tremor rating score reduction after conversion to LCP-Tacro in patients experiencing severe hand tremors.