Figures & data
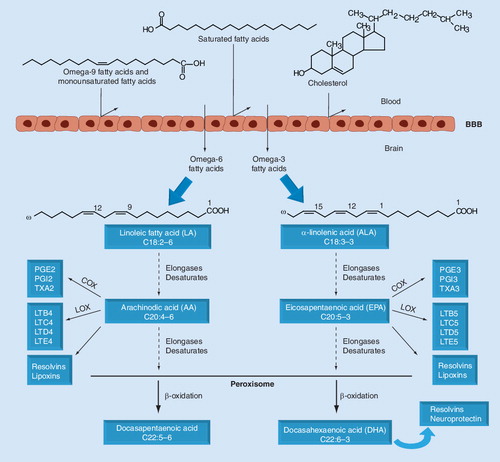
LA and ALA are converted to long-chain fatty acids through reactions of desaturation and elongation. The synthesis of DHA requires the passage of precursor fatty acids into the peroxisome, where they suffer one cycle of β-oxidation to produce DHA. The AA and EPA fatty acids synthesize prostanoids and leukotrienes by the enzymes COX and LOX, respectively. AA, EPA and DHA can also synthesize resolvins, proteins that have neuroprotective functions. These reactions occur primarily in the liver, but they can also take place in the brain, once omega-3 and omega-6 are transferred by the BBB.
AA: Arachinodic acid; ALA: α-linolenic acid; COX: Cyclooxygenase; DHA: Docasahexaenoic acid; EPA: Eicosapentaenoic acid; LA: Linoleic acid; LTB4: Leukotriene B4; LTB5: Leukotriene B5; LTC4: Leukotriene C4; LTC5: Leukotriene C5; LTD4: Leukotriene D4; LTD5: Leukotriene D5; LTE4: Leukotriene E4; LTE5: Leukotriene E5; LOX: Lipoxygenase; PGE2: Prostaglandin 2; PGE3: Prostaglandin 3; PGI2: Prostacyclin I2; PGI3: Prostacyclin I3; TXA2: Thromboxane A2; TXA3: Thromboxane A3.
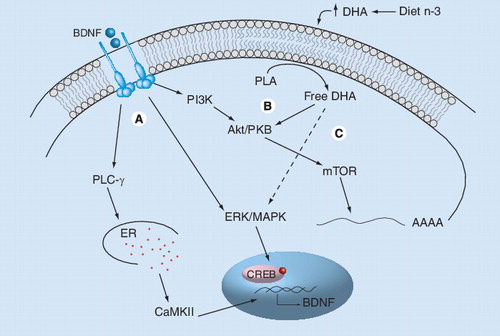
(A) Mature BDNF binds to the TrkB receptor and activates three main intracellular signaling pathways involving PLC-γ, ERK/MAPK and Akt/PKB. Activation of PLC-γ leads to the release of calcium from the ER and to the activation of CaMKII, leading to the phosphorilation of CREB and activation of gene transcription. Activation of the ERK/MAPK pathway can also regulate transcription through the phosphorylation of CREB, whereas PI3K phosphorylates and activates Akt/PKB and mTOR, regulating translation initiation. (B) DHA increases neurotrophic signaling by activating one branch of the classical BDNF signaling via PI3-K/Akt pathways. (C) DHA increases BDNF synthesis by activating MAPK signaling. Activated MAPK phosphorylates CREB, which translocates into the nucleus and activates BDNF gene transcription.
BDNF: Brain-derived neurotrophic factor; CaMKII: Calcium–calmodulin kinase II; CREB: cAMP response element-binding protein; DHA: Docosahexaenoic acid; ER: Endoplasmic reticulum; n-3: Omega-3 fatty acids; PLA: Phospholipase A2; PLC-γ: Phospholipase C γ; TrkB: Tyrosine kinase receptor B.
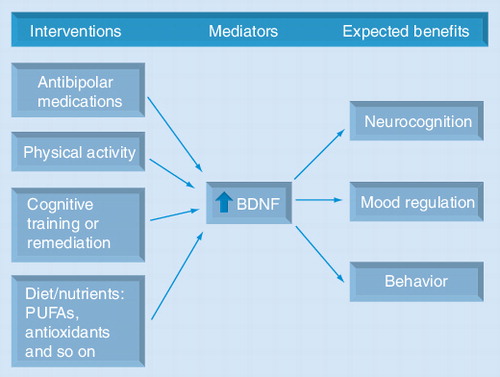
Several pharmacological agents used to treat bipolar disorders, such as mood stabilizers, antidepressants and atypical antipsychotics, increase BDNF levels. Interventions on lifestyle-related factors, such as regular physical exercise and diet/nutrition, also increase neurotrophins. In addition, the neurocognitive benefits derived from psychosocial interventions, such as cognitive training or remediation, are thought to be mediated by neurotrophins. These strategies may have synergistic effects. Multitargeted interventions that increase BDNF and other neutrophins may maximize neurogenesis/neuroprotection, and as a result potentially improve mood, neurocognitive functioning and mental health. The expected benefits from each intervention may be different, but in all instances BDNF would be a putative key mediator.
BDNF: Brain-derived neurotrophic factor; PUFA: Polyunsaturated fatty acid.