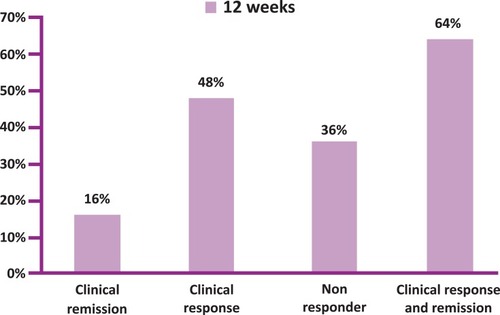
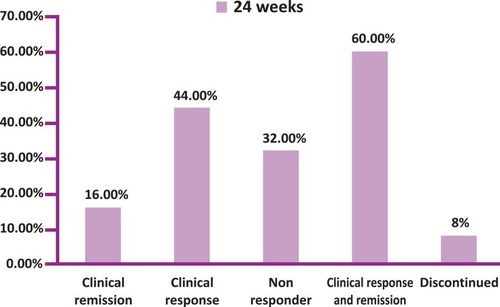
Figures & data
Table 1 Mayo Scoring System
Table 2 Baseline And Demographic Patient Characteristics
Figure 1 Study design in ulcerative colitis patients on ADA biosimilar and reasons for discontinuation.
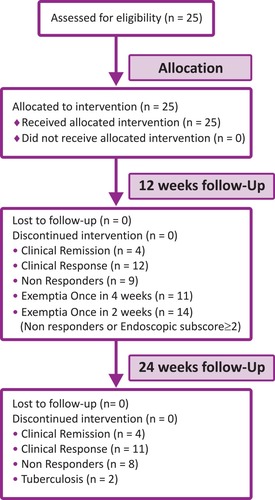
Figure 4 Total Mayo Score (TMS) at Baseline, 12weeks and 24 weeks (N=25 at 12 weeks and N=23 at 24 weeks).
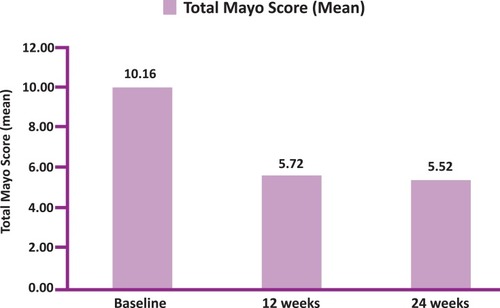
Figure 5 Mayo endoscopic sub-score (MESS) at Baseline, 12weeks and 24 weeks (N=25 at 12 weeks and N=23 at 24 weeks).
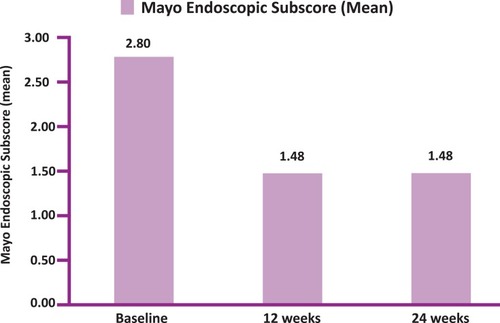
Table 3 Total Mayo Score (TMS) And Mayo Endoscopic Sub-Score (MESS) At Baseline, 12 Weeks And 24 Weeks (N= 25 At 12 Weeks And N=23 At 24 weeks)
Table 4 Total Mayo Score (TMS) And Mayo Endoscopic Sub-Score (MESS) Of 14 Patients Whose Dose Has Been Accelerated At 12 Weeks From Once In 4 Weeks To Once In 2 Weeks
Table 5 Various Reported Real-Time Studies On Adalimumab In UC