Figures & data
Table 1 Demographics/characteristics of MONARCH sarilumab and adalimumab patient populations (base case analysis) and MOBILITY aggregate patient population (individual patient simulation)
Table 2 Treatment response rates applied to short-term and long-term models
Figure 1 Model structure for long-term analysis.
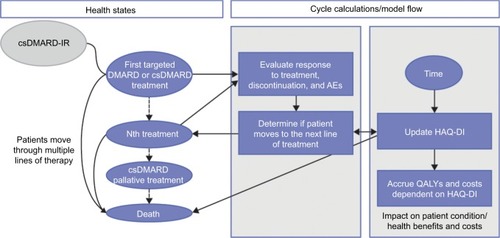
Table 3 Drug costs
Figure 2 Responders (%) and NNT.
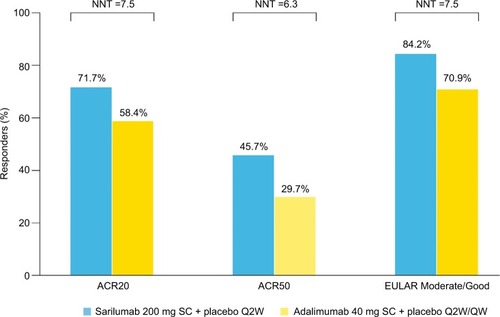
Figure 3 Cost per responder at 24 weeks: (A) base case analysis and (B) sensitivity analyses.
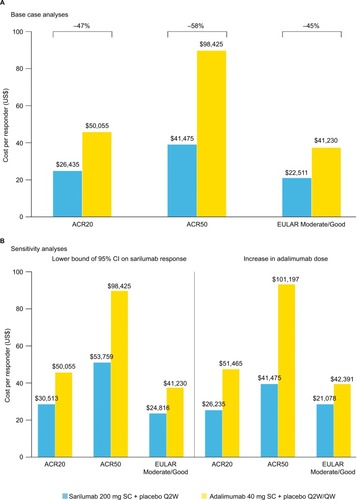
Figure 4 Long-term incremental analyses.
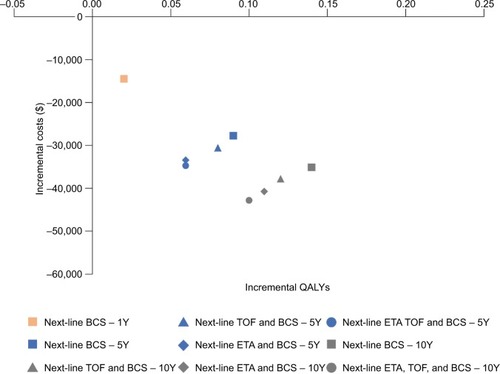
Table 4 Long-term analyses: costs
Table 5 Long-term analyses: outcomes

