Figures & data
Table 1 Dosing Regimens of Rituximab in All Approved Indications
Figure 1 The estimation model and weights for the budget impact analysis study. Weightings were calculated according to the following formula: where j is the incidence rate of indication j. consumption per year j is the total need of milligrams for the treatment of indication j. rituximab treatment rate is the proportion of patients treated in indication j treated with rituximab.
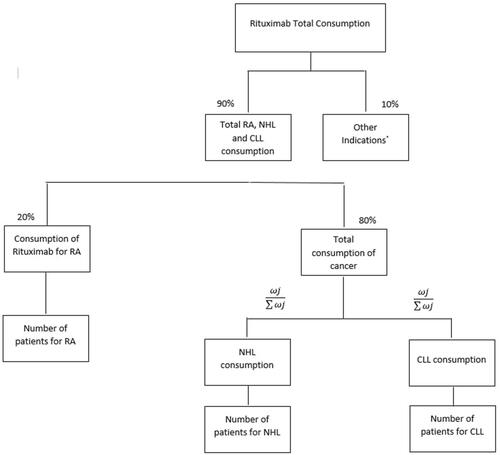
Table 2 A Projection of Total Budget Impact Savings Related to the Introduction of a Rituximab Biosimilar Over a 1-Year Period in Addition to the Number of Patients That Would Benefit from Gaining Accessibility to Rituximab Treatment
