Figures & data
Figure 1 Overview of the relationships between ALSFRS-R, mortality and treatment.
Abbreviation: ALSFRS-R, revised amyotrophic lateral sclerosis functional rating scale.
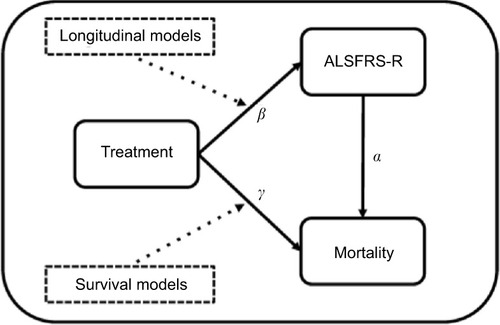
Table 1 Null and alternative hypothesis of each analytical strategy to evaluate the combined treatment effect on survival and functional loss
Figure 2 Rates of functional decline and mortality in the PRO-ACT database with a simulated treatment scenario.
Abbreviations: ALSFRS-R, revised amyotrophic lateral sclerosis functional rating scale; PRO-ACT, Pooled Resource Open-Access ALS Clinical Trials.
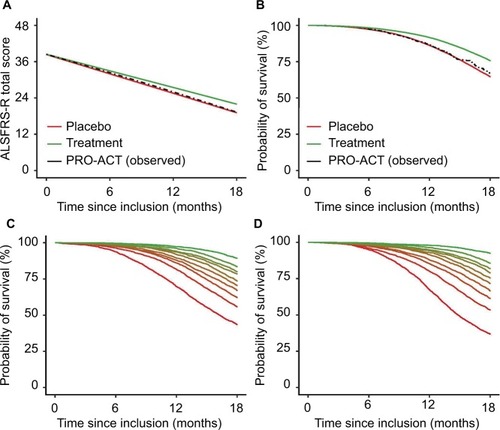
Table 2 Baseline characteristics of the PRO-ACT database’s placebo patients
Table 3 Empirical power of each strategy for trials with a maximum follow-up duration of 18 months
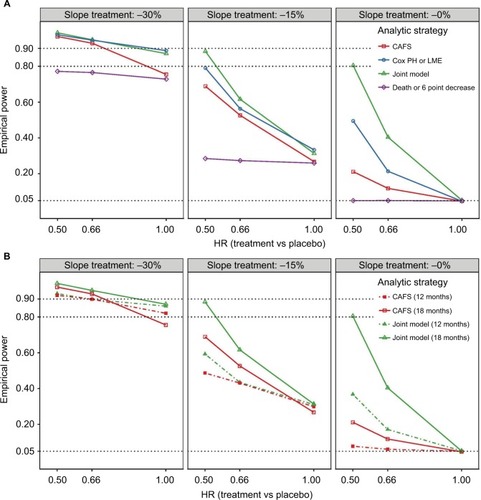
Figure 3 Empirical power of the four analytical strategies for different treatment scenarios.
Abbreviations: ALSFRS-R, revised amyotrophic lateral sclerosis functional rating scale; CAFS, combined assessment of function and survival; HR, hazard ratio; LME, linear mixed effects; PH, proportional hazard.