Figures & data
Table 1 The Frequency of the Four Gene Mutations Among Epithelial Ovarian Cancer
Figure 1 Mutational landscape of epithelial ovarian cancer. (A) Mutations in significantly mutated genes in epithelial ovarian cancer and selected known oncogenes and tumor suppressors. Genes mutations are shown in subtype of epithelial ovarian cancer. (B) Variants for P53, BRCA1/2, PIK3CA, and KRAS, color-coded by subtype of epithelial ovarian cancer. Splice site mutations are indicated as involving the acceptor site (exon – nucleotide position of mutation).
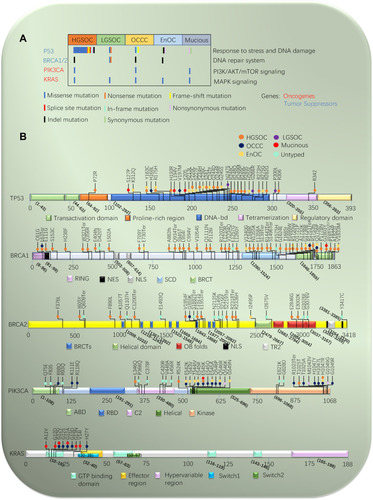
Figure 2 Cellular functions of P53 in ovarian cancer. P53 is activated to regulate the expression downstream genes to induce a series of cellular responses, such as cell cycle arrest, apoptosis, senescence, autophagy, DNA repair, angiogenesis, migration and metabolism in different types of extracellular and intracellular stress (eg, nutrient deprivation, telomere erosion, hypoxia, DNA damage, ribosomal stress, and oncogene activation). The primary treatment strategy for patients with ovarian cancer with P53 mutations is focused on restoring WT-P53 function to mutant P53, Blocking the interaction of WT P53 with MDM2/MDM4, and gene therapy with P53.
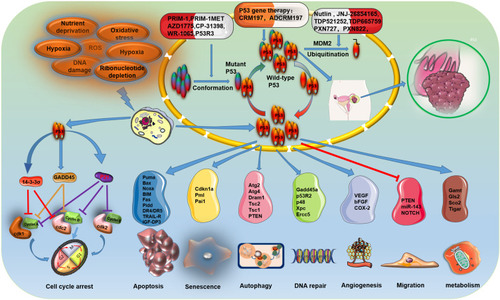
Table 2 Trials Using Target Agents on Gene Mutation in Ovarian Cancer
Figure 3 The mechanisms of PARP inhibitors in BRCA-related ovarian cancer. PARP-1 mediates the repair of SSBs through the activation and recruitment of repair enzymes. Counterclockwise: activated PARP-1 detects damaged SSB in DNA and binds to adjacent DNA. Once bound, PARP-1 catalyzes the cleavage of the coenzyme nicotinamide adenine dinucleotide (NAD +) to nicotinamide and ADP-ribose to produce a highly charged branch of high poly (ADP-ribose) (PAR). Repair proteins are recruited to the site of injury to repair damaged DNA. After finishing repair, the PAR chain is degraded by PAR glycohydrolase (PARG). BRCA1/2 genes, located on chromosomes 17 (17q21) and 13 (13q12.3), play an important role in regulating cell cycle and the DNA repair system. The mutated BRCA gene loses its function of repairing DNA, and PARP inhibitors also inhibit the repair of DNA by PARP, thereby promoting tumor cell death.
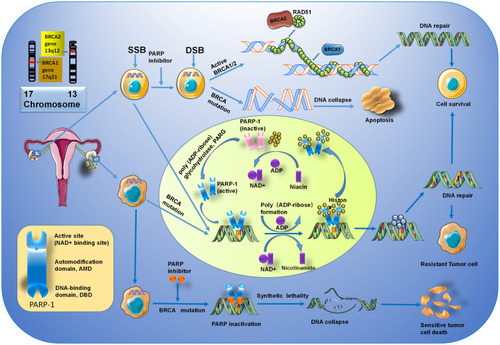
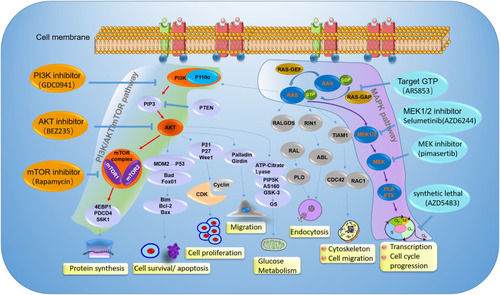
Figure 4 The mechanisms of PI3K/Akt/mTOR pathway and MAPK pathway, and inhibitors in ovarian cancer clinical development. Illustration the therapy strategy via inhibiting the PI3K/Akt/mTOR pathway (green) and MAPK pathway (amaranth) in ovarian cancer patients with PIK3CA and KRAS gene mutation. The orange represents a different inhibitory effect of repressing tumor growth by targeting different sites on the PI3K/Akt/mTOR pathway. For patients with PIK3CA gene mutation, clinical treatment drugs are mainly divided into PI3K inhibitor, AKT inhibitor, and mTOR inhibitor. The blue represents a different inhibitor target MAPK pathway in ovarian cancer patients with KRAS gene mutation. The therapy strategy includes restricting KRAS bound to GTP and targeting its downstream signaling pathway.